Advances in Green and Sustainable Photo Redox Catalysis for Organic Transformation Photocatalysis
Main Article Content
Abstract
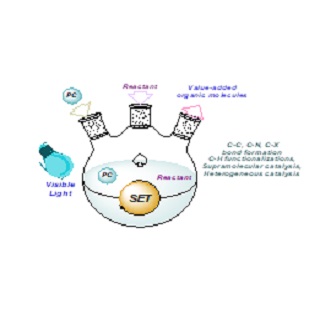
Advances in visible-light-mediated photo redox catalysis have created an exceptional approach for small-molecule activation and new chemical bond formation in organic synthesis. This is an alternative technique to generate new reactive intermediates and enable distinct synthetic strategies that were previously unthinkable. Of the many trademarks, photochemistry is often classified as “green” technology, promoting organic reactions under mild conditions without the necessity for potent and wasteful solvents, oxidants and reductants. This mini review covers the overview of the primary principles of photoredox catalysis, discussing various metal-based and organophotocatalysts. It further surveys a variety of reactions, including trifluoromethylation, cyanation, C-H functionalizations, supramolecular catalysis, and heterogeneous catalysis in both aqueous and alcoholic mediums.
Article Details
How to Cite
References
J. Liu, L. Lu, D. Wood, S. Lin. New Redox Strategies in Organic Synthesis by Means of Electrochemistry and Photochemistry. ACS Cent. Sci., 2020, 6, 1317. https://doi.org/10.1021/acscentsci.0c00549
M. Bera, D. S. Lee, E. J. Cho. Advances in N-centered intermediates by energy transfer. photocatalysis, Trends Chem., 2021, 3,877. https://doi.org/10.1016/j.trechm.2021.06.001
C. S. Wang, P. H. Dixneuf, J. F. Soulé. Photoredox Catalysis for Building C–C Bonds from C(sp2)–H Bonds. Chem. Rev., 2018, 118, 7532. https://doi.org/10.1021/acs.chemrev.8b00077
G. E. M. Crisenza, P. Melchiorre. Chemistry glows green with photoredox catalysis. Nat. Commun., 2020, 11, 803. https://doi.org/10.1038/s41467-019-13887-8
T. P. Yoon, M. A Ischay, J. Du. Visible light photocatalysis as a greener approach to photochemical synthesis. Nat. Chem., 2010, 2, 527. https://doi.org/10.1038/nchem.687
T. Ghosh, T. Slanina, B. König. Visible light photocatalytic reduction of aldehydes by Rh(III)–H: a detailed mechanistic study.Chem. Sci., 2015, 6, 2027. https://doi.org/10.1039/C4SC03709J
A. Call, C. Casadevall, F. Acuña-Parés, A. Casitas, J. Lloret-Fillol. Dual cobalt–copper light-driven catalytic reduction of aldehydes and aromatic ketones in aqueous media.Chem. Sci., 2017, 8, 4739. https://doi.org/10.1039/C7SC01276D
Y. Hou, P. Wan. Formal intramolecular photoredox chemistry of anthraquinones in aqueous solution: photodeprotection for alcohols, aldehydes and ketones. Photochem. Photobiol. Sci., 2008, 7, 588. https://doi.org/10.1039/B718970B
Y. Hou, L. A. Huck, P. Wan. Long-range intramolecular photoredox reaction via coupled charge and proton transfer of triplet excited anthraquinones mediated by water. Photochem. Photobiol. Sci., 2009, 8, 1408. https://doi.org/10.1039/B909479B
H. Hou, S. Zhu, F. Pan, M. Rueping. Visible-light photoredox-catalyzed synthesis of nitrones: unexpected rate acceleration by water in the synthesis of isoxazolidines. Org. Lett., 2014, 16, 2872. https://doi.org/10.1021/ol500893g
M. Goez, M. Schiewek, M. HO Musa. Near‐UV Photoionization of [Ru (bpy) 3]2+: A Catalytic Cycle with an Excited Species as Catalyst. Angew. Chem. Int. Ed., 2002, 41, 1535. https://doi.org/10.1002/1521-3773(20020503)41:9<1535::AID-ANIE1535>3.0.CO;2-1
M. Goez, D. V. Ramin-Marro, M. H. O. Musa, M. Schiewek. Photoionization of [Ru (bpy) 3]2+: A catalytic cycle with water as sacrificial donor. J. Phys. Chem. A, 2004, 108, 1090. https://doi.org/10.1021/jp036790r
Z. J. Xu, D. Xu, X. Zheng, F. Wang, B. Tan, Y. Hu, Y. Lv, G. Zhong. Diastereoselective HOTf-catalyzed three-component one-pot 1,3-dipolar cycloaddition of α-diazo ester, nitrosobenzene and electron-deficient alkene. Chem. Comm., 2010, 46, 2504. https://doi.org/10.1039/B924575H
A. J. J. Lennox, G. C. L. Jones. Organotrifluoroborate Hydrolysis: Boronic Acid Release Mechanism and an Acid–Base Paradox in Cross-Coupling. J. Am. Chem. Soc., 2012, 134, 7431. https://doi.org/10.1021/ja300236k
J. J. Dai, W. M. Zhang, Y. J. Shu, Y. Y. Sun, J. Xu, Y. S. Feng, H. J. Xu. Deboronative cyanation of potassium alkyltrifluoroborates via photoredox catalysis. Chem. Comm., 2016, 52, 6793. https://doi.org/10.1039/C6CC01530A
E. Speckmeier, P. J. W. Fuchs, K. Zeitler. A synergistic LUMO lowering strategy using Lewis acid catalysis in water to enable photoredox catalytic, functionalizing C–C cross-coupling of styrenes. Chem. Sci., 2018, 9, 7096. https://doi.org/10.1039/C8SC02106F
R. A. Aycock, H. Wang, N. T. Jui. A mild catalytic system for radical conjugate addition of nitrogen heterocycles.Chem. Sci., 2017, 8, 3121. https://doi.org/10.1039/C7SC00243B
F. Chen, X-H Xu, F-L Qing. Photoredox-catalyzed addition of dibromofluoromethane to alkenes: direct synthesis of 1-bromo-1-fluoroalkanes.Org. Lett, 2021, 23, 2364. https://doi.org/10.1021/acs.orglett.1c00639
D. Xue, Z. H. Jia, C. J. Zhao, Y. Y. Zhang, C. Wang, J. Xiao. Direct Arylation of N-Heteroarenes with Aryldiazonium Salts by Photoredox Catalysis in Water. Chem. Eur. J., 2014, 20, 2960. https://doi.org/10.1002/chem.201304120
J. Zhang, J. Chen, X. Zhang, X. Lei. Total Syntheses of Menisporphine and Daurioxoisoporphine C Enabled by Photoredox-Catalyzed Direct C–H Arylation of Isoquinoline with Aryldiazonium Salt. J. Org. Chem., 2014, 79, 10682. https://doi.org/10.1021/jo5020432
H. Qiao, S. Sun, F. Yang, Y. Zhu, J. Kang, Y. Wu, Y. Wu. Merging Photoredox Catalysis with Iron (III) Catalysis: C5‐H Bromination and Iodination of 8‐Aminoquinoline Amides in Water. Adv. Synth. Catal., 2017, 359, 1976. https://doi.org/10.1002/adsc.201601053
D. Jespersen, B. Keen, J. I. Day, A. Singh, J. Briles, D. Mullins, J. D. Weaver III. Solubility of Iridium and Ruthenium Organometallic Photoredox Catalysts. Org. Process Res. Dev., 2018, 23, 1087. https://doi.org/10.1021/acs.oprd.9b00041
R. C. W. van Lier, A. D. de Bruijn, G. Roelfes. A Water-Soluble Iridium Photocatalyst for Chemical Modification of Dehydroalanines in Peptides and Proteins. Chem. Eur. J., 2021, 27, 1430. https://doi.org/10.1002/chem.202002599
T-TH. Nguyen, C. J. O’Brien, M. LN. Tran, S. H. Olson, N. S. Settineri, S. B. Prusiner, N. A. Paras, J. Conrad. Water-soluble iridium photoredox catalyst for the trifluoromethylation of biomolecule substrates in phosphate buffered saline solvent.Org. Lett., 2021, 23, 3823. https://doi.org/10.1021/acs.orglett.1c00871
D. Brusselle, P. Bauduin, L. Girard, A. Zaulet, C. Viñas, F. Teixidor, I. Ly, O. Diat. Lyotropic lamellar phase formed from monolayered θ-shaped carborane-cage amphiphiles. Angew. Chem. Int. Ed., 2013, 52, 12114. https://doi.org/10.1002/anie.201307357
M. Tarres, C. Vinas, P. González‐Cardoso, M. M. Hänninen, R. Sillanpää, V. Ďorďovič, M. Uchman, F. Teixidor, P. Matějíček. Aqueous self‐assembly and cation selectivity of cobaltabisdicarbollidedianionic dumbbells. Chem. Eur. J., 2014, 20, 6786. https://doi.org/10.1002/chem.201402193
M. Uchman, V. Ďorďovič, Z. Tošner, P. Matějíček. Classical amphiphilic behavior of nonclassical amphiphiles: a comparison of metallacarborane self‐assembly with SDS micellization. Angew. Chem., 2015, 127, 14319. https://doi.org/10.1002/ange.201506545
I. Guerrero, Z. Kelemen, C. Viñas, I. Romero, F. Teixidor. Metallacarboranes as Photoredox Catalysts in Water, Metallacarboranes as Photoredox Catalysts in Water. Chem. Eur. J., 2020, 26, 5027. https://doi.org/10.1002/chem.201905395
I. Guerrero, C. Viñas, I. Romero, F. Teixido. A stand-alone cobalt bis (dicarbollide) photoredox catalyst epoxidates alkenes in water at extremely low catalyst load. Green Chem., 2021, 23, 10123. https://doi.org/10.1039/D1GC03119H
I. Guerrero, A. Saha, J. A. M. Xavier, Clara Vinas, I. Romero, F. Teixidor. Noncovalently linked metallacarboranes on functionalized magnetic nanoparticles as highly efficient, robust, and reusable photocatalysts in aqueous medium. ACS Appl. Mater. Interfaces., 2020,12, 56372. https://doi.org/10.1021/acsami.0c17847
G. Troyano, I., C. Viñas, X. Fontrodona, I. R. García, F. T. Bombardó. Aqueous Persistent Noncovalent Ion-Pair Cooperative Coupling in a Ruthenium Cobaltabis (dicarbollide) System as a Highly Efficient Photoredox Oxidation Catalyst. Inorg. Chem., 2021, 60, 8898. https://doi.org/10.1021/acs.inorgchem.1c00751
S. Srinath, R. Abinaya, A. Prasanth, M. Mariappan, R. Sridhar, B. Baskar. Reusable, homogeneous water soluble photoredox catalyzed oxidative dehydrogenation of N-heterocycles in a biphasic system: application to the synthesis of biologically active natural products.Green Chem., 2020, 22, 2575. https://doi.org/10.1039/D0GC00569J
M-j. Bu, C. Cai, F. Gallou, B. H. Lipshutz. PQS-enabled visible-light iridium photoredox catalysis in water at room temperature. Green Chem, 2018, 20, 1233. https://doi.org/10.1039/C7GC03866F
N. A. Romero, D. A. Nicewicz. Organic photoredox catalysis. Chem. Rev.,2016, 17, 10075. https://doi.org/10.1021/acs.chemrev.6b00057
D. P. Hari, B. König. Synthetic applications of eosin Y in photoredox catalysis. Chem. Comm. 2014, 50. 6688. https://doi.org/10.1039/C4CC00751D
K. A. Margrey, D. A. Nicewicz. A general approach to catalytic alkene anti-Markovnikov hydrofunctionalization reactions via acridinium photoredox catalysis.Acc. Chem. Res., 2016, 49, 1997. https://doi.org/10.1021/acs.accounts.6b00304
M. Majek, A. J. V. Wangelin. Mechanistic perspectives on organic photoredox catalysis for aromatic substitutions. Acc. Chem. Res., 2016, 49, 2316. https://doi.org/10.1021/acs.accounts.6b00293
M-j. Bu, G-p. Lu., J. Jiang, C. Cai. Merging visible-light photoredox and micellar catalysis: arylation reactions with anilines nitrosated in situ. Catal. Sci. Technol., 2018, 8, 3728. https://doi.org/10.1039/C8CY01221K
L. J. Gooßen, N. Rodríguez, K. Gooßen. Carboxylic acids as substrates in homogeneous catalysis. Angew. Chem. Int. Ed., 2008, 47, 3100. https://doi.org/10.1002/anie.200704782
W-P. Mai, G. Song, G-C. Sun, L-R. Yang, J-W. Yuan, Y-M. Xiao, P. Mao, L. –B. Qu. Cu/Ag-catalyzed double decarboxylative cross-coupling reaction between cinnamic acids and aliphatic acids in aqueous solution. RSC Adv., 2013, 3, 19264. https://doi.org/10.1039/C3RA43144D
H. Yang, H. Yan, P. Sun, Y. Zhu, L. Lu, D. Liu, G. Rong, J. Mao. Iron-catalyzed direct alkenylation of sp 3 (C–H) bonds via decarboxylation of cinnamic acids under ligand-free conditions. Green Chem, 2013, 15, 976. https://doi.org/10.1039/C3GC37131J
H. Huang, K. Jia, Y. Chen. Hypervalent Iodine Reagents Enable ChemoselectiveDeboronative/ DecarboxylativeAlkenylation by Photoredox Catalysis.Angew. Chem., 2015, 127, 1901. https://doi.org/10.1002/ange.201410176
H. Huang, G. Zhang, Y. Chen. Dual Hypervalent Iodine(III) Reagents and Photoredox Catalysis Enable Decarboxylative Ynonylation under Mild Conditions.Angew. Chem., 2015, 127, 7983. https://doi.org/10.1002/ange.201502369
S. Mizuta, K. M. Engle, S. Verhoog, O. G. López, M. O’Duill, M. Médebielle, K. Wheelhouse, G. Rassias, A. L. Thompson, V. Gouverneur. Trifluoromethylation of allylsilanes under photoredox catalysis. Org. Lett., 2013,15, 1250 .https://doi.org/10.1021/ol400184t
D. K. Kölmel, R. P. Loach, T. Knauber, M. E. Flanagan. Employing Photoredox Catalysis for DNA‐Encoded Chemistry: Decarboxylative Alkylation of α‐Amino Acids. ChemMedChem, 2018, 13, 2159. https://doi.org/10.1002/cmdc.201800492
D. K. Kölmel, J. Meng, M-H. Tsai, J. Que, R. P. Loach, T. Knauber, J. Wan, M. E. Flanagan. On-DNA decarboxylative arylation: merging photoredox with nickel catalysis in water. ACS Comb. Sci., 2019, 21, 588. https://doi.org/10.1021/acscombsci.9b00076
N. Noto, Y. Hyodo, M. Yoshizawa, T. Koike, M. Akita. Transition Metal-Free Supramolecular Photoredox Catalysis in Water: A Phenoxazine Photocatalyst Encapsulated in V-Shaped Aromatic Amphiphiles.ACS Catal., 2020, 10, 14283. https://doi.org/10.1021/acscatal.0c04221
D. Kalyani, K. B. McMurtrey, S. R. Neufeldt, M. S. Sanford. Room-temperature C–H arylation: merger of Pd-catalyzed C–H functionalization and visible-light Photocatalysis. J. Am. Chem. Soc., 2011, 133, 18566.https://doi.org/10.1021/ja208068w
L. Liang, M-S. Xie, H-X. Wang, H-Y. Niu, G-R. Qu, H-M. Guo. Visible-light-mediated monoselectiveortho C–H arylation of 6-arylpurine nucleosides with diazonium salts. J. Org. Chem. 2017, 82, 5966.https://doi.org/10.1021/acs.joc.7b00659
J. Jiang, W-M. Zhang, J-J. Dai, J. Xu, H-J. Xu. Visible-light-promoted C–H arylation by merging palladium catalysis with organic photoredox catalysis. J. Org. Chem., 2017, 82, 3622. https://doi.org/10.1021/acs.joc.7b00140
D. Xue, Z‐H. Jia, C‐J. Zhao, Y‐Y. Zhang, C. Wang, J. Xiao. Direct Arylation of N‐Heteroarenes with Aryldiazonium Salts by Photoredox Catalysis in Water. Chem. Eur. J., 2014, 20, 2960. https://doi.org/10.1002/chem.201304120
T. McCallum, L. Barriault. Direct alkylation of heteroarenes with unactivatedbromoalkanes using photoredox gold catalysis. Chem. Sci, 2016, 7, 4754. https://doi.org/10.1039/C6SC00807K
S. R. Kandukuri, A. Bahamonde, I. Chatterjee, I. D. Jurberg, E. C. Escudero‐Adán, P. Melchiorre. X‐Ray Characterization of an Electron Donor–Acceptor Complex that Drives the Photochemical Alkylation of Indoles.Angew. Chem. Int. Ed., 2015, 54, 1485. https://doi.org/10.1002/anie.201409529
J. Jin, D. W. C. MacMillan. Direct α-Arylation of Ethers through the Combination of Photoredox-Mediated C-H Functionalization and the Minisci Reaction.Angew. Chem., 2015, 127, 1585. https://doi.org/10.1002/ange.201410432
W. Fu, F. Xu, Y. Fu, M. Zhu, J. Yu, C. Xu, D. Zou. Synthesis of 3, 3-disubstituted oxindoles by visible-light-mediated radical reactions of aryl diazonium salts with N-arylacrylamides. J. Org. Chem., 2013, 78, 12202. https://doi.org/10.1021/jo401894b