Composites of Chitosan for Biomedical Applications Biomaterials
Main Article Content
Abstract
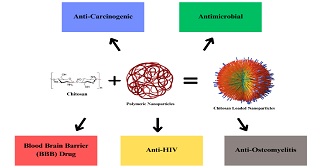
Chitosan (CS) is a cationic polysaccharide that consists of jumble distributed units of N-acetyl-D-glucosamine (acetylated unit) and β-(1→4)-linked D-glucosamine (deacetylated unit). CS has gained significant importance in the field of biomedicine due to its non-toxicity, biodegradability, and biocompatibility properties. It has numerous potential applications, including in the development of bandages that can reduce bleeding and serve as antibacterial agents, as well as DDSs that can transport medication across the skin and BBB. CS can be used alone or in amalgamation with antibiotics and extracts to create antimicrobial wound dressings that are effective in treating infections. Overall, CS and its derivatives hold great promise for biomedical implicatives, particularly in wound healing and DD. Due to its qualities, CS-based NPs are being studied as possible DDS against diseases like Leishmaniasis, Bacterial Diseases, and Cancer. Throughout the chapter we will have an overview of these properties of CS, their possible applications in the biomedical field and their possible role against these diseases.
Article Details
How to Cite
References
J. Lakkakula, D. Divakaran, R. Srivastava, P. Ingle, A. Gade, R. Raut. In Situ Growth of Biocompatible Biogenic Silver Nanoparticles in Poly-Vinyl Alcohol Thin Film Matrix. IEEE Trans Nanobioscience., 2023, 22, 480. https://doi.org/10.1109/TNB.2022.3208310
T. Arfin, F. Mohammad. Chemistry and structural aspects of chitosan towards biomedical applications, In: S. Ikram, S. Ahmed (Eds.), Natural polymers: derivatives, blends and composite, Vol.1, Nova Science Publishers, New York, 2016, pp. 265-280.
J. Lakkakula, G. K. P. Srilekha, P. Kalra, S. A. Varshini, S. Penna. Exploring the promising role of chitosan delivery systems in breast cancer treatment: A comprehensive review. Carbohydr Res., 2024, 545, 109271. https://doi.org/10.1016/J.CARRES.2024.109271
A. Kumar, W. Abbas, G. Herbein. HIV-1 latency in monocytes/macrophages. Viruses, 2014, 6, 1837. https://doi.org/10.3390/V6041837
C. W. Norden, E. Kennedy. Experimental osteomyelitis. I. A description of the model. J. Infect. Dis., 1970, 122, 410. https://doi.org/10.1093/INFDIS/122.5.410
P. D. Potdar, A. U. Shetti. Evaluation of anti-metastatic effect of chitosan nanoparticles on esophageal cancer-associated fibroblasts. J. Cancer Metastasis Treat., 2016, 2, 259. https://doi.org/10.20517/2394-4722.2016.25
I. A. Mayer, V. G. Abramson, B. D. Lehmann, J. A. Pietenpol. New strategies for triple-negative breast cancer—deciphering the heterogeneity. Clin. Cancer Res., 2014, 20, 782. https://doi.org/10.1158/1078-0432.ccr-13-0583
U. Bickel, T. Yoshikawa, W. M. Pardridge. Delivery of peptides and proteins through the blood-brain barrier. Adv. Drug Deliv. Rev., 2001, 46, 247. https://doi.org/10.1016/S0169-409X(00)00139-3
T. D. Azad, J. Pan, I. D. Connolly, A. Remington, C. M. Wilson, G. A. Grant. Therapeutic strategies to improve drug delivery across the blood-brain barrier. Neurosurg. Focus., 2015, 38, 9. https://doi.org/10.3171/2014.12.FOCUS14758
T. Arfin. Chitosan and its derivatives: overlook of commercial application in diverse field, In: S. Ahmed, S. Ikram (Eds.), Chitosan: derivatives, composites and applications, Scrivener Publishing LLC, Beverly, 2017, pp.115-150. https://doi.org/10.1002/9781119364849
F. Mohammad, T. Arfin, H. A. Al-Lohedan. Enhanced biological activity and biosorption performance of trimethyl chitosan-loaded cerium oxide particles. J. Ind. Eng. Chem., 2017, 45, 33. https://doi.org/10.1016/J.JIEC.2016.08.029
T. Arfin. Current innovative chitosan-based water treatment of heavy metals: A sustainable approach, In: S. Ahmed, S. Kanchi, G. Kumar (Eds.), Handbook of biopolymers: advances and multifaceted applications, Jenny Stanford Publishing, Singapore, 2018, pp. 167-182. https://doi.org/10.1201/9780429024757-7
S. S. Waghmare, T. Arfin. Defluoridation by adsorption with chitin-chitosan-alginate-polymers-cellulose-resins-algae and fungi-A Review. IRJET, 2015, 2, 1179. https://api.semanticscholar.org/CorpusID:212470446
Y. Zhu, C. Goh, A. Shrestha. Biomaterial Properties Modulating Bone Regeneration. Macromol. Biosci., 2021, 21, 2000365. https://doi.org/10.1002/mabi.202000365
X. Zhao, J. L. Pathak, W. Huang, C. Zhu, Y. Li, H. Guan, S. Zeng, L. Ge, Y. Shu. Metformin enhances osteogenic differentiation of stem cells from human exfoliated deciduous teeth through AMPK pathway. J. Tissue Eng. Regen. Med., 2020, 14, 1869. https://doi.org/10.1002/term.3142
J. J. Elsner, I. Berdicevsky, M Zilberman. In vitro microbial inhibition and cellular response to novel biodegradable composite wound dressings with controlled release of antibiotics. Acta Biomater., 2011, 7, 325. https://doi.org/10.1016/j.actbio.2010.07.013
J. Lakkakula, P. Kalra, G. Mallick, H. Mittal, I. Uddin. Revolutionizing cancer treatment: Enhancing photodynamic therapy with cyclodextrin nanoparticles and synergistic combination therapies. Materials Today Sustainability, 2024, 28, 100958. https://doi.org/10.1016/J.MTSUST.2024.100958
A. P. Sughanthy Siva, M. N. M. Ansari. A Review on Bone Scaffold Fabrication Methods. IRJET, 2015, 2, 1232.
Y. Xu, Y. Liu, Q. Liu, S. Lu, X. Chen, W. Xu, F. Shi. Co-delivery of bufalin and nintedanib via albumin sub-microspheres for synergistic cancer therapy. J. Control. Release, 2021, 338, 705. https://doi.org/10.1016/J.JCONREL.2021.08.049
B. J. Grattan, H. C. Freake. Zinc and Cancer: Implications for LIV-1 in Breast Cancer. Nutrients, 2012, 4, 648. https://doi.org/10.3390/NU4070648
S. Jaiswal, P. K. Dutta, S. Kumar, J. Koh, S. Pandey. Methyl methacrylate modified chitosan: Synthesis, characterization and application in drug and gene delivery. Carbohydr Polym., 2019, 211, 109. https://doi.org/10.1016/J.CARBPOL.2019.01.104
L. T. W. Hin, R. Subramaniam. Congestion control of heavy vehicles using electronic road pricing: The Singapore experience. Int. J. Heavy Veh. Syst., 2006, 13, 37. https://doi.org/10.1504/IJHVS.2006.009116
R. Vivek, V. Nipun Babu, R. Thangam, K. S. Subramanian, S. Kannan. PH-responsive drug delivery of chitosan nanoparticles as Tamoxifen carriers for effective anti-tumor activity in breast cancer cells. Colloids Surf. B Biointerfaces., 2013, 111, 117. https://doi.org/10.1016/j.colsurfb.2013.05.018
W. J. Yu, J. M. Son, J. Lee, S. –H. Kim, I. –C. Lee, H. –S. Baek, I. –S. Shin, C. Moon, S. –H. Kim, J. –C. Kim. Effects of silver nanoparticles on pregnant dams and embryo-fetal development in rats. Nanotoxicology, 2013, 8, 85. https://doi.org/10.3109/17435390.2013.857734
S. J. Lee, H. S. Min, S. H. Ku, S. Son, I. C. Kwon, S. H. Kim, K. Kim. Tumor-targeting glycol chitosan nanoparticles as a platform delivery carrier in cancer diagnosis and therapy. Nanomedicine, 2014, 9, 1697. https://doi.org/10.2217/NNM.14.99
S. S. Kim, A. Rait, E. Kim, K. F. Pirollo, M. Nishida, N. Farkas, J. A. Dagata, E. H. Chang. A nanoparticle carrying the p53 gene targets tumors including cancer stem cells, sensitizes glioblastoma to chemotherapy and improves survival. ACS Nano., 2014, 8, 5494. https://doi.org/10.1021/NN5014484
H. Park, K. Park, D. Kim. Preparation and swelling behavior of chitosan-based superporous hydrogels for gastric retention application. J. Biomed. Mater. Res. A., 2006, 76, 144. https://doi.org/10.1002/JBM.A.30533
S. Hattori, H. Fujisaki, T. Kiriyama, T. Yokoyama, S. Irie. Real-time zymography and reverse zymography: A method for detecting activities of matrix metalloproteinases and their inhibitors using FITC-labeled collagen and casein as substrates. Anal. Biochem., 2002, 301, 27. https://doi.org/10.1006/abio.2001.5479
H. Zhang, Y. Zhao. Preparation, characterization and evaluation of tea polyphenol-Zn complex loaded β-chitosan nanoparticles. Food Hydrocoll., 2015, 48, 260. https://doi.org/10.1016/j.foodhyd.2015.02.015
P. Deshpande, A. Dapkekar, M. D. Oak, K. M. Paknikar, J. M. Rajwade. Zinc complexed chitosan/TPP nanoparticles: A promising micronutrient nanocarrier suited for foliar application. Carbohydr. Polym., 2017, 165, 394. https://doi.org/10.1016/j.carbpol.2017.02.061
S. F. Rodrigues, L. A. Fiel, A. L. Shimada, N. R. Pereira, S. S. Guterres, A. R. Pohlmann, S. H. Farskyl. Lipid-core nanocapsules act as a drug shuttle through the blood brain barrier and reduce glioblastoma after intravenous or oral administration. J. Biomed. Nanotechnol., 2016,12, 986. https://doi.org/10.1166/JBN.2016.2215
X. Wang, Y. Du, H. Liu. Preparation, characterization and antimicrobial activity of chitosan-Zn complex. Carbohydr. Polym., 2004, 56, 21. https://doi.org/10.1016/j.carbpol.2003.11.007
P. –H. Chang, K. Sekine, H. –M. Chao, S. –H. Hsu, E. Chern. Chitosan promotes cancer progression and stem cell properties in association with Wnt signaling in colon and hepatocellular carcinoma cells. Sci Rep., 2017, 7, 1. https://doi.org/10.1038/srep45751
P. C. Srinivasa, M. N. Ramesh, K. R. Kumar, R. N. Tharanathan. Properties of chitosan films prepared under different drying conditions. J. Food Eng., 2004, 63, 79. https://doi.org/10.1016/S0260-8774(03)00285-1
W. Chen, Y. Li, S. Yang, L. Yue, Q. Jiang, W. Xia. Synthesis and antioxidant properties of chitosan and carboxymethyl chitosan-stabilized selenium nanoparticles. Carbohydr. Polym. 2015, 132, 574. https://doi.org/10.1016/J.CARBPOL.2015.06.064
C. T. Lin, C. Y. Chen, S. G. Chen, T. M. Chen, S. C. Chang. Preserve the lower limb in a patient with calcaneal osteomyelitis and severe occlusive peripheral vascular disease by partial calcanectomy. J. Med. Sci., 2015, 35, 74. https://doi.org/10.4103/1011-4564.156016
J. Q. Gao, Q. Q. Zhao, T. F. Lv, W. –P. Shuai, J. Zhou, G. –P. Tang, W. –Q. Liang, Y. Tabata, Y. –L. Hu. Gene-carried chitosan-linked-PEI induced high gene transfection efficiency with low toxicity and significant tumor-suppressive activity. Int. J. Pharm., 2010, 387, 286. https://doi.org/10.1016/j.ijpharm.2009.12.033
T. Pitt. Management of antimicrobial-resistant Acinetobacter in hospitals. Nurs. Stand., 2007, 21, 51. https://doi.org/10.7748/NS2007.05.21.35.51.C4556
X. Sun, Z. Wang, H. Kadouh, K. Zhou. The antimicrobial, mechanical, physical and structural properties of chitosan-gallic acid films. LWT., 2014, 57, 83. https://doi.org/10.1016/j.lwt.2013.11.037
N. A. Mohamed, N. Y. Al-mehbad. Novel terephthaloyl thiourea cross-linked chitosan hydrogels as antibacterial and antifungal agents. Int. J. Biol. Macromol., 2013, 57, 111. https://doi.org/10.1016/j.ijbiomac.2013.03.007
M. Mohseni, A. Shamloo, Z. Aghababaie, H. Afjoul, S. Abdi, H. Moravvej, M. Vossoughi. A comparative study of wound dressings loaded with silver sulfadiazine and silver nanoparticles: In vitro and in vivo evaluation. Int J. Pharm., 2019, 564, 350. https://doi.org/10.1016/j.ijpharm.2019.04.068
C. M. de Moura, J. M. de Moura, N. M. Soares, L. A. de Almeida Pinto. Evaluation of molar weight and deacetylation degree of chitosan during chitin deacetylation reaction: Used to produce biofilm. CEP:PI., 2011, 50, 351. https://doi.org/10.1016/J.CEP.2011.03.003
A. Muñoz-Bonilla, C. Echeverria, Á. Sonseca, M. P. Arrieta, M. Fernández-García. Bio-based polymers with antimicrobial properties towards sustainable development. Materials. 2019, 12, 641. https://doi.org/10.3390/MA12040641
S. K. Nandi, P. Mukherjee, S. Roy, B. Kundu, D. K. De, D. Basu. Local antibiotic delivery systems for the treatment of osteomyelitis - A review. Mater. Sci. Eng. C., 2009, 29, 2478. https://doi.org/10.1016/j.msec.2009.07.014
J. K. Smith, J. D. Bumgardner, H. S. Courtney, M. S. Smeltzer, W. O. Haggard. Antibiotic-loaded chitosan film for infection prevention: A preliminary in vitro characterization. J. Biomed. Mater. Res. B Appl Biomater., 2010, 94, 203. https://doi.org/10.1002/JBM.B.31642
N. Monteiro, M. Martins, A. Martins, Nuno A Fonseca, J. N. Moreira, R. L. Reis, N. M. Neves. Antibacterial activity of chitosan nanofiber meshes with liposomes immobilized releasing gentamicin. Acta Biomater., 2015, 18, 196. https://doi.org/10.1016/J.ACTBIO.2015.02.018
C. H. Wang, C. W. Chang, C. A. Peng. Gold nanorod stabilized by thiolated chitosan as photothermal absorber for cancer cell treatment. J. Nanopar. Res. 2011,13, 2749. https://doi.org/10.1007/s11051-010-0162-5
W. Shao, H. Liu, J. Wu, S. Wang, X. Liu, M. Huang, P. Xu. Preparation, antibacterial activity and pH-responsive release behavior of silver sulfadiazine loaded bacterial cellulose for wound dressing applications. J. Taiwan Inst. Chem. Eng., 2016, 63, 404. https://doi.org/10.1016/j.jtice.2016.02.019
Y. Cheng, F. Yang, K. Zhang, Y. Zhang, Y. Cao, C. Liu, H. Lu, H. Dong, X. Zhang. Non-Fenton-Type Hydroxyl Radical Generation and Photothermal Effect by Mitochondria-Targeted WSSe/MnO2 Nanocomposite Loaded with Isoniazid for Synergistic Anticancer Treatment. Adv. Funct. Mater., 2019, 29, 1903850. https://doi.org/10.1002/ADFM.201903850
R. M. Wang, N. P. He, P. F. Song, Y. F. He, L. Ding, Z. Q. Lei. Preparation of nano-chitosan Schiff-base copper complexes and their anticancer activity. Polym. Adv. Technol., 2009, 20, 959. https://doi.org/10.1002/pat.1348
A. Piegat, A. Żywicka, A. Niemczyk, A. Goszczyńska. Antibacterial Activity of N,O-Acylated Chitosan Derivative. Polymers, 2021, 13, 107. https://doi.org/10.3390/POLYM13010107
W. M. Pardridge. Drug transport across the blood-brain barrier. J. Cereb. Blood Flow Metab., 2012, 32, 1959. https://doi.org/10.1038/JCBFM.2012.126
L. Wen, Y. Tan, S. Dai, Y. Zhu, T. Meng, X. Yang, Y. Liu, X. Liu, H. Yuan, F. Hu. Vegf-mediated tight junctions pathological fenestration enhances doxorubicin-loaded glycolipid-like nanoparticles traversing bbb for glioblastoma-targeting therapy. Drug Deliv., 2017, 24, 1843. https://doi.org/10.1080/10717544.2017.1386731
N. J. Abbott, A. A. K. Patabendige, D. E. M. Dolman, S. R. Yusof, D. J. Begley. Structure and function of the blood-brain barrier. Neurobiol. Dis., 2010, 37, 13. https://doi.org/10.1016/J.NBD.2009.07.030
R. Daneman, A. Prat. The blood–brain barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, a020412. https://doi.org/10.1101/cshperspect.a020412
S. Wohlfart, S. Gelperina, J. Kreuter. Transport of drugs across the blood-brain barrier by nanoparticles. J. Controlled Release, 2012, 161, 264. https://doi.org/10.1016/j.jconrel.2011.08.017
J. Freiherr, M. Hallschmid, W. H. Frey, Y. F. Brünner, C. D. Chapman, C. Hölscher, S. Craft, F. G. De Felice, C. Benedict. Intranasal insulin as a treatment for alzheimer’s disease: A review of basic research and clinical evidence. CNS Drugs, 2013, 27, 505. https://doi.org/10.1007/S40263-013-0076-8
Y. Chen, S. Feng, W. Liu, Z. Yuan, P. Yin, F. Gao. Vitamin E succinate-grafted-chitosan oligosaccharide/RGD-conjugated TPGS mixed micelles loaded with paclitaxel for U87MG tumor therapy. Mol Pharm., 2017, 14, 1190. https://doi.org/10.1021/ACS.MOLPHARMACEUT.6B01068
M. D. Shadab, R. A. Khan, G. Mustafa, K. Chuttani, S. Baboota, J. K. Sahni, J. Ali. Bromocriptine loaded chitosan nanoparticles intended for direct nose to brain delivery: Pharmacodynamic, Pharmacokinetic and Scintigraphy study in mice model. Eur. J. Pharm. Sci., 2013, 48, 393. https://doi.org/10.1016/J.EJPS.2012.12.007
Z. Zhao, S. Lou, Y. Hu, J. Zhu, C. Zhang. A Nano-in-Nano Polymer-Dendrimer Nanoparticle-Based Nanosystem for Controlled Multidrug Delivery. Mol. Pharm., 2017, 14, 2697. https://doi.org/10.1021/ACS.MOLPHARMACEUT.7B00219
L. Gao, X. Wang, J. Ma, D. Hao, P. Wei, L. Zhou, G. Liu. Evaluation of TPGS-modified thermo-sensitive Pluronic PF127 hydrogel as a potential carrier to reverse the resistance of P-gp-overexpressing SMMC-7721 cell lines. Colloids Surf B Biointerfaces, 2016, 140, 307. https://doi.org/10.1016/j.colsurfb.2015.12.057
J. Rip, L Chen, R Hartman, A. van den Heuvel, A. Reijerkerk, J. van Kregten, B. van der Boom, C. Appeldoorn, M. de Boer, D. Maussang, E. C. M. de Lange, P. J. Gaillard. Glutathione PEGylated liposomes: Pharmacokinetics and delivery of cargo across the blood-brain barrier in rats. J. Drug Target., 2014, 22, 460. https://doi.org/10.3109/1061186X.2014.888070
M. Salvalaio, L Rigon, D Belletti, F. D'Avanzo, F. Pederzoli, B. Ruozi, O. Marin, M. A. Vandelli, F. Forni, M. Scarpa, R. Tomanin, G. Tosi. Targeted polymeric nanoparticles for brain delivery of high molecular weight molecules in lysosomal storage disorders. PLoS One, 2016, 11, e0156452. https://doi.org/10.1371/JOURNAL.PONE.0156452
A Dalpiaz, G Paganetto, B Pavan, M. Fogagnolo, A. Medici, S. Beggiato, D. Perrone. Zidovudine and ursodeoxycholic acid conjugation: Design of a new prodrug potentially able to bypass the active efflux transport systems of the central nervous system. Mol Pharm., 2012, 9, 957. https://doir.org/10.1021/MP200565G
I. N. Khan, S. Navaid, W. Waqar, D. Hussein, N. Ullah, M. U. A. Khan, Z. Hussain, A. Javed. Chitosan-Based Polymeric Nanoparticles as an Efficient Gene Delivery System to Cross Blood Brain Barrier: In Vitro and In Vivo Evaluations. Pharmaceuticals, 2024, 17, 169. https://doi.org/10.3390/PH17020169
T. Banerjee, S. Mitra, A. K. Singh, R. K. Sharma, A. Maitra. Preparation, characterization and biodistribution of ultrafine chitosan nanoparticles. Int. J. Pharm., 2002, 243, 93. https://doi.org/10.1016/S0378-5173(02)00267-3
J. Li, K. Kataoka. Chemo-physical Strategies to Advance the in Vivo Functionality of Targeted Nanomedicine: The Next Generation. J. Am. Chem. Soc. 2021,143, 538. https://doi.org/10.1021/JACS.0C09029
D. Carradori, C. Balducci, F. Re, D. Brambilla, B. L. Droumaguet, O. Flores, A. Gaudin, S. Mura, G. Forloni, L. O. -Gutierrez, F. Wandosell, M. Masserini, P. Couvreur, J. Nicolas, K. Andrieux. Antibody-functionalized polymer nanoparticle leading to memory recovery in Alzheimer’s disease-like transgenic mouse model. Nanomedicine, 2018, 14, 609. https://doi.org/10.1016/J.NANO.2017.12.006
A. E. Caprifico, P. J. S. Foot, E. Polycarpou, G. Calabrese. Overcoming the blood-brain barrier: Functionalised chitosan nanocarriers. Pharmaceutics, 2020, 12, 1013. https://doi.org/10.3390/PHARMACEUTICS12111013
S. Debaisieux, F. Rayne, H. Yezid, B. Beaumelle. The Ins and Outs of HIV-1 Tat. Traffic., 2012, 13, 355. https://doi.org/10.1111/J.1600-0854.2011.01286.X
A. Dev, N. S. Binulal, A. Anitha, S. V. Nair, T. Furuike, H. Tamura, R. Jayakumar. Preparation of poly(lactic acid)/chitosan nanoparticles for anti-HIV drug delivery applications. Carbohydr. Polym., 2010, 80, 833. https://doi.org/10.1016/J.CARBPOL.2009.12.040
J. S. Park, Y. W. Cho. In vitro cellular uptake and cytotoxicity of paclitaxel-loaded glycol chitosan self-assembled nanoparticles. Macromol. Res., 2007, 15, 513. https://doi.org/10.1007/BF03218824
L. N. Ramana, S. Sharma, S. Sethuraman, U. Ranga, U. M. Krishnan. Investigation on the stability of saquinavir loaded liposomes: Implication on stealth, release characteristics and cytotoxicity. Int. J. Pharm., 2012, 431, 120. https://doi.org/10.1016/j.ijpharm.2012.04.054
H. Y. Nam, S. M. Kwon, H. Chung, S. –Y. Lee, S. –H. Kwon, H. Jeon, Y. Kim, J. H. Park, J. Kim, S. Her, Y. –K. Oh, I. C. Kwon, K. Kim, S. Y. Jeong. Cellular uptake mechanism and intracellular fate of hydrophobically modified glycol chitosan nanoparticles. J. Control. Release. 2009, 135, 259. https://doi.org/10.1016/j.jconrel.2009.01.018
S. D. Gioia, A. Trapani, D. Mandracchia, E. D. Giglio, S. Cometa, V. Mangini, F. Arnesano, G. Belgiovine, S. Castellani, L. Pace, M. A. Lavecchia, G. Trapani, M. Conese, G. Puglisi, T. Cassano. Intranasal delivery of dopamine to the striatum using glycol chitosan/sulfobutylether-β-cyclodextrin based nanoparticles. Eur. J. Pharm. Biopharm., 2015, 94, 180. https://doi.org/10.1016/j.ejpb.2015.05.019
R. Rozana, Y. Yulizar, A. Saefumillah, D. O. B. Apriandanu. Synthesis, characterization and in vitro release study of efavirenz-loaded chitosan nanoparticle. AIP Conf. Proc., 2020, 2242, 040004. https://doi.org/10.1063/5.0007923
K. Chaturvedi, K. Ganguly, M. N. Nadagouda, T. M. Aminabhavi. Polymeric hydrogels for oral insulin delivery. J. Control. Release, 2013, 165,129. https://doi.org/10.1016/j.jconrel.2012.11.005
B. Singh, B. Garg, S. C. Chaturvedi, S. Arora, R. Mandsaurwale, R. Kapil, B. Singh. Formulation development of gastroretentive tablets of lamivudine using the floating-bioadhesive potential of optimized polymer blends. J. Pharm. Pharmacol., 2012, 64, 654. https://doi.org/10.1111/J.2042-7158.2011.01442.X
D. A. Cobb, N. Smith, S. Deodhar, A. N. Bade, N. Gautam, B. L. D. Shetty, J. McMillan, Y. Alnouti, S. M. Cohen, H. E. Gendelman, B. Edagwa. Transformation of tenofovir into stable ProTide nanocrystals with long-acting pharmacokinetic profiles. Nat. Commun., 2021, 12, 5458. https://doi.org/10.1038/S41467-021-25690-5
S. A. Agnihotri, N. N. Mallikarjuna, T. M. Aminabhavi. Recent advances on chitosan-based micro- and nanoparticles in drug delivery. J. Control. Release., 2004, 100, 5. https://doi.org/10.1016/j.jconrel.2004.08.010
S. Sharma, A. Tyagi, S. Dang. Nose to Brain Delivery of Transferrin conjugated PLGA nanoparticles for clonidine. Int. J. Biol. Macromol. 2023, 252, 126471. https://doi.org/10.1016/j.ijbiomac.2023.126471
L. N. Ramana, S. Sharma, S. Sethuraman, U. Ranga, U. M. Krishnan. Evaluation of chitosan nanoformulations as potent anti-HIV therapeutic systems. Biochim. Biophys Acta., 2014, 1840, 476. https://doi.org/10.1016/J.BBAGEN.2013.10.002
D. Soundararajan, L. N. Ramana, P. Shankaran, U. M. Krishnan. Nanoparticle-based strategies to target HIV-infected cells. Colloids Surf B Biointerfaces., 2022, 213, 112405. https://doi.org/10.1016/j.colsurfb.2022.112405
F. Karadeniz, S. K. Kim. Chapter three - antidiabetic activities of chitosan and its derivatives: a mini review. Adv. Food Nutr. Res. 2014, 73, 33. https://doi.org/10.1016/B978-0-12-800268-1.00003-2
(a) N. Jaber, M. Al-Remawi, F. Al-Akayleh, N. Al-Muhtaseb, I. S. I. Al-Adham, P. J. Collier. A review of the antiviral activity of Chitosan, including patented applications and its potential use against COVID-19. J Appl Microbiol. 2022, 132, 41. https://doi.org/10.1111/jam.15202
(b) N. Hsan, S Kumar, J. Koh, and P. K Dutta. Chitosan modified multi-walled carbon nanotubes and arginine aerogel for enhanced carbon capture. Int. J. Biol. Macromol., 2023, 252, 126523. https://doi.org/10.1016/j.ijbiomac.2023.126523
P. Heydari, M. Kharaziha, J. Varshosaz, A. Z. Kharazi, S. H. Javanmard. Co-release of nitric oxide and L-arginine from poly (β-amino ester)-based adhesive reprogram macrophages for acceleratedwound healing and angiogenesis in vitro and in vivo. Biomater. Adv., 2024, 158. 213762. https://doi.org/10.1016/j.bioadv.2024.213762
Y. Zhang, X. Shen, P. Ma, Z. Peng, K. Cai. Composite coatings of Mg-MOF74 and Sr-substituted hydroxyapatite on titanium substrates for local antibacterial, anti-osteosarcoma and pro-osteogenesis applications. Mater. Lett., 2019, 241, 18. https://doi.org/10.1016/j.matlet.2019.01.033
C. Makarov, V. Cohen, A. Raz-Pasteur, I. Gotman. In vitro elution of vancomycin from biodegradable osteoconductive calcium phosphate-polycaprolactone composite beads for treatment of osteomyelitis. Eur. J. Pharm. Sci., 2014, 62, 49. https://doi.org/10.1016/j.ejps.2014.05.008
N. H. Radwan, M. Nasr, R. A. H. Ishak, N. F. Abdeltawab, G. A. S. Awad. Chitosan-calcium phosphate composite scaffolds for control of post-operative osteomyelitis: Fabrication, characterization, and in vitro–in vivo evaluation. Carbohydr. Polym. 2020, 244, 116482. https://doi.org/10.1016/j.carbpol.2020.116482
R. A. A. Muzzarelli. Chitosan composites with inorganics, morphogenetic proteins and stem cells, for bone regeneration. Carbohydr. Polym., 2011, 83, 1433. https://doi.org/10.1016/j.carbpol.2010.10.044
R. A. A. Muzzarelli. Genipin-crosslinked chitosan hydrogels as biomedical and pharmaceutical aids. Carbohydr. Polym., 2009, 77, 1. https://doi.org/10.1016/j.carbpol.2009.01.016
K. E. Beenken, J. K. Smith, R. A. Skinner, S. G. Mclaren, W. Bellamy, M. J. Gruenwald, H. J. Spencer, J. A. Jennings, W. O. Haggard, M. S. Smeltzer. Chitosan coating to enhance the therapeutic efficacy of calcium sulfate-based antibiotic therapy in the treatment of chronic osteomyelitis. J. Biomater. Appl., 2014, 29, 514. https://doi.org/10.1177/0885328214535452
V. Uskoković, C. Hoover, M. Vukomanović, D. P. Uskoković, T. A. Desai. Osteogenic and antimicrobial nanoparticulate calcium phosphate and poly-(d,l-lactide-co-glycolide) powders for the treatment of osteomyelitis. Mater. Sci. Eng. C., 2013, 33, 3362. https://doi.org/10.1016/j.msec.2013.04.023
V. Pawar, R. Srivastava. Chitosan-polycaprolactone blend sponges for management of chronic osteomyelitis: A preliminary characterization and in vitro evaluation. Int. J. Pharm., 2019, 568, 118553. https://doi.org/10.1016/j.ijpharm.2019.118553
V. Uskoković, T. A. Desai. In vitro analysis of nanoparticulate hydroxyapatite/chitosan composites as potential drug delivery platforms for the sustained release of antibiotics in the treatment of osteomyelitis. J. Pharm. Sci., 2014, 103, 567. https://doi.org/10.1002/jps.23824
N. H. Radwan, M. Nasr, R. A. H. Ishak, N. F. Abdeltawab, G. A. S. Awad. Chitosan-calcium phosphate composite scaffolds for control of post-operative osteomyelitis: Fabrication, characterization, and in vitro–in vivo evaluation. Carbohydr. Polym., 2020, 244, 116482. https://doi.org/10.1016/J.CARBPOL.2020.116482
E Cevher, Z Orhan, L Mülazimoǧlu, D. Sensoy, M. Alper, A. Yildiz, Y. Ozsoy. Characterization of biodegradable chitosan microspheres containing vancomycin and treatment of experimental osteomyelitis caused by methicillin-resistant Staphylococcus aureus with prepared microspheres. Int J. Pharm., 2006, 317, 127. https://doi.org/10.1016/j.ijpharm.2006.03.014
P. Shi, Y. Zuo, X. Li, Q. Zou, H. Liu, L. Zhang, Y. Li, Y. S. Morsi. Gentamicin-impregnated chitosan/nanohydroxyapatite/ethyl cellulose microspheres granules for chronic osteomyelitis therapy. J. Biomed. Mater. Res. A., 2010, 93A, 1020. https://doi.org/10.1002/JBM.A.32598
V. Pawar, R. Srivastava. Layered assembly of chitosan nanoparticles and alginate gel for management of post-surgical pain and infection. 16th International Conference on Nanotechnology - IEEE NANO., 2016, 241. https://doi.org/10.1109/NANO.2016.7751388
D. Słota, J. Jampilek, A. Sobczak-Kupiec. Targeted Clindamycin Delivery Systems: Promising Options for Preventing and Treating Bacterial Infections Using Biomaterials. Int. J. Mol. Sci., 2024, 25, 4386. https://doi.org/10.3390/ijms25084386
R. A. Hashad, R. A. H. Ishak, A. S. Geneidi, S. Mansour. Surface functionalization of methotrexate-loaded chitosan nanoparticles with hyaluronic acid/human serum albumin: Comparative characterization and in vitro cytotoxicity. Int. J. Pharm., 2017, 522, 128. https://doi.org/10.1016/j.ijpharm.2017.03.008