Insights on metal-free type I photosensitizers for photodynamic therapy Chemical Biology
Main Article Content
Abstract
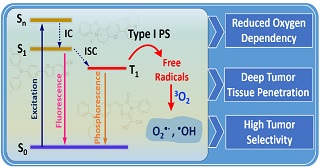
Non-invasive treatment techniques have drawn a lot of interest due to the rising need for precise and secure cancer treatment. One such treatment method is photodynamic therapy (PDT), which uses the light irradiation of photosensitizers (PSs) to produce reactive oxygen species (ROS), which kill cancer cells. Most of the conventional photosensitizers used in the PDT process rely on molecular oxygen to produce cytotoxic ROS, known by the name of type II PSs. Because type II PSs requires oxygen to produce ROS, their full potential is not realized in hypoxic tumor tissues. On the other hand, type I PSs can increase the effectiveness of PDT in hypoxic tumor tissues since they rely less on oxygen to produce ROS. Consequently, it has become increasingly crucial to develop type I PSs to treat hypoxic malignancies. Numerous type I PSs of inorganic origin have been developed so far. Nonetheless, certain issues like poor biodegradability and persistent toxicity exist. Type I PSs based on organic compounds were developed in response to these concerns since they are comparatively more biocompatible and biodegradable. Therefore, in this article, we describe recent developments in the development of organic type I PSs for the PDT.
Article Details
How to Cite
References
(a) C. M. Moore, D. Pendse, M. Emberton. Photodynamic therapy for prostate cancer—a review of current status and future promise. Nat. Rev. Urol. 2009, 6, 18-30. DOI: https://doi.org/10.1038/ncpuro1274 (b) W. Fan, P. Huang, X. Chen. Overcoming the Achilles' heel of photodynamic therapy. Chem. Soc. Rev. 2016, 45, 6488. https://doi.org/10.1039/C6CS00616G
(a) R. Ackroyd, C. Kelty, N. Brown, M. Reed. The history of photodetection and photodynamic therapy. Photochem. Photobiol. 2001, 74, 656. https://doi.org/10.1562/0031-8655(2001)0740656THOPAP2.0.CO2
J. D. Spikes. The origin and meaning of the term “photodynamic” (as used in “photodynamic therapy”, for example). J. Photochem. Photobiol. B Biol.1991, 9, 369. https://doi.org/10.1016/1011-1344(91)80172-E
J. P. Celli, B. Q. Spring, I. Rizvi, C. L. Evans, K. S. Samkoe, S. Verma, B. W. Pogue, T. Hasan. Imaging and Photodynamic Therapy: Mechanisms, Monitoring, and Optimization. Chem. Rev. 2010, 110, 2795. https://doi.org/10.1021/cr900300p
V. P. Chauhan, R. K. Jain. Strategies for advancing cancer nanomedicine. Nat. Mater. 2013, 12, 958. https://doi.org/10.1038/nmat3792
(a) W. Fan, B. Yung, P. Huang, X. Chen. Nanotechnology for Multimodal Synergistic Cancer Therapy. Chem. Rev. 2017, 117, 13566. https://doi.org/10.1021/acs.chemrev.7b00258 (b) P. Yang, S. Gai, J. Lin. Functionalized mesoporous silica materials for controlled drug delivery. Chem. Soc. Rev. 2012, 41, 3679. https://doi.org/10.1039/C2CS15308D
(a) L. B. Josefsen, R. W. Boyle. Unique Diagnostic and Therapeutic Roles of Porphyrins and Phthalocyanines in Photodynamic Therapy, Imaging and Theranostics. Theranostics 2012, 2, 916. https://doi.org/10.7150/thno.4571 (b) K. Han, S. -B. Wang, Q. Lei, J. -Y. Zhu, X. -Z. Zhang. Ratiometric Biosensor for Aggregation-Induced Emission-Guided Precise Photodynamic Therapy. ACS Nano 2015, 9, 10268. https://doi.org/10.1021/acsnano.5b04243 (c) T. C. Pham, V. -N. Nguyen, Y. Choi, S. Lee, J. Yoon. Recent Strategies to Develop Innovative Photosensitizers for Enhanced Photodynamic Therapy. Chem. Rev. 2021, 121, 13454. https://doi.org/10.1021/acs.chemrev.1c00381
C. S. Foote. Definition of Type I and Type II Photosensitized Oxidation. Photochem. Photobiol. 1991, 54, 659. https://doi.org/10.1111/j.1751-1097.1991.tb02071.x
H. Shi, P. J. Sadler. How promising is phototherapy for cancer? Br. J. Cancer 2020,123, 871. https://doi.org/10.1038/s41416-020-0926-3
J. Moan, S. Sommer. Oxygen Dependence of the Photosensitizing Effect of Hematoporphyrin Derivative in NHIK 3025 Cells. CancerRes.1985, 45, 1608.
(a) Y. Cheng, H. Cheng, C. Jiang, X. Qiu, K. Wang, W. Huan, A. Yuan, J. Wu, Y. Hu. Perfluorocarbon nanoparticles enhance reactive oxygen levels and tumour growth inhibition in photodynamic therapy. Nat. Commun. 2015, 6, 8785. https://doi.org/10.1038/ncomms9785 (b) J. Kim, H. R. Cho, H. Jeon, D. Kim, C. Song, N. Lee, S. H. Choi, T. Hyeon. Continuous O2-Evolving MnFe2O4 Nanoparticle-Anchored Mesoporous Silica Nanoparticles for Efficient Photodynamic Therapy in Hypoxic Cancer. J. Am. Chem. Soc. 2017, 139, 10992. https://doi.org/10.1021/jacs.7b05559
(a) K. Plaetzer, B. Krammer, J. Berlanda, F. Berr, T. Kiesslich. Photophysics and photochemistry of photodynamic therapy: fundamental aspects. Laser. Med. Sci. 2008, 24, 259. https://doi.org/10.1007/s10103-008-0539-1 (b) A. Kamkaew, S. H. Lim, H. B. Lee, L. V. Kiew, L. Y. Chung and K. Burgess. BODIPY dyes in photodynamic therapy. Chem. Soc. Rev. 2013, 42, 77. https://doi.org/10.1039/C2CS35216H
Y. Li, W. Zhang, J. Niu, Y. Chen. Mechanism of Photogenerated Reactive Oxygen Species and Correlation with the Antibacterial Properties of Engineered Metal-Oxide Nanoparticles. ACS Nano 2012, 6,5164. https://doi.org/10.1021/nn300934k
(a) X. Li, D. Lee, J. -D. Huang, J. Yoon. Phthalocyanine-Assembled Nanodots as Photosensitizers for Highly Efficient Type I Photoreactions in Photodynamic Therapy. Angew. Chem. Int. Ed. 2018, 57, 9885. https://doi.org/10.1002/anie.201806551 (b) Z. Lv, H. Wei, Q. Li, X. Su, S. Liu, K. Y. Zhang, W. Lv, Q. Zhao, X. Li, W. Huang. Achieving efficient photodynamic therapy under both normoxia and hypoxia using cyclometalated Ru(ii) photosensitizer through type I photochemical process. Chem. Sci. 2018, 9, 502. https://doi.org/10.1039/C7SC03765A (c) J. S. Nam, M.-G. Kang, J. Kang, S.-Y. Park, S. J. C. Lee, H.-T. Kim, J. K. Seo, O.-H. Kwon, M. H. Lim, H.-W. Rhee, T.-H. Kwon. Endoplasmic Reticulum-Localized Iridium(III) Complexes as Efficient Photodynamic Therapy Agents via Protein Modifications. J. Am. Chem. Soc. 2016, 138, 10968. https://doi.org/10.1021/jacs.6b05302 (d) S. S. Lucky, N. M. Idris, Z. Li, K. Huang, K. C. Soo, Y. Zhang. Titania Coated Upconversion Nanoparticles for Near-Infrared Light Triggered Photodynamic Therapy, ACS Nano 2015, 9, 191. https://doi.org/10.1021/nn503450t
Y. Cai, W. Si, W. Huang, P. Chen, J. Shao, X. Dong. Organic Dye Based Nanoparticles for Cancer Phototheranostics. Small 2018, 14, 1704247. https://doi.org/10.1002/smll.201704247
(a) H. Abrahamse, M. R. Hamblin. New photosensitizers for photodynamic therapy. Biochem. J. 2016, 473, 347. https://doi.org/10.1042/BJ20150942 (b) X. Zheng, U. W. Sallum, S. Verma, H. Athar, C. L. Evans, T. Hasan. Exploiting a Bacterial Drug-Resistance Mechanism: A Light-Activated Construct for the Destruction of MRSA. Angew. Chem. Int. Ed. 2009, 48, 2148. https://doi.org/10.1002/anie.200804804 (c) O. J. Klein, H. Yuan, N. H. Nowell, C. Kaittanis, L. Josephson, C. L. Evans. An Integrin-Targeted, Highly Diffusive Construct for Photodynamic Therapy. Sci. Rep. 2017, 7, 13375. https://doi.org/10.1038/s41598-017-13803-4
M. Li, J. Xia, R. Tian, J. Wang, J. Fan, J. Du, S. Long, X. Song, J. W. Foley, X. Peng. Near-Infrared Light-Initiated Molecular Superoxide Radical Generator: Rejuvenating Photodynamic Therapy against Hypoxic Tumors. J. Am. Chem. Soc. 2018, 140, 14851. https://doi.org/10.1021/jacs.8b08658
M. Li, T. Xiong, J. Du, R. Tian, M. Xiao, L. Guo, S. Long, J. Fan, W. Sun, K. Shao, X. Song, J. W. Foley, X. Peng. Superoxide Radical Photogenerator with Amplification Effect: Surmounting the Achilles’ Heels of Photodynamic Oncotherapy. J. Am. Chem. Soc. 2019, 141, 2695. https://doi.org/10.1021/jacs.8b13141
M. Li, Y. Shao, J. H. Kim, Z. Pu, X. Zhao, H. Huang, T. Xiong, Y. Kang, G. Li, K. Shao, J. Fan, J. W. Foley, J. S. Kim, X. Peng. Unimolecular Photodynamic O2-Economizer to Overcome Hypoxia Resistance in Phototherapeutics. J. Am. Chem. Soc. 2020, 142, 5380. https://doi.org/10.1021/jacs.0c00734
Z. Yi, X. Qin, L. Zhang, H. Chen, T. Song, Z. Luo, T. Wang, J. Lau, Y. Wu, T. B. Toh, C.-S. Lee, W. Bu, X. Liu. Mitochondria-Targeting Type-I Photodrug: Harnessing Caspase-3 Activity for Pyroptotic Oncotherapy. J. Am. Chem. Soc. 2024, 146, 9413. https://doi.org/10.1021/jacs.4c01929
Y. Zhang, M. Zhao, J. Miao, W. Gu, J. Zhu, B. Cheng, Q. Li, Q. Miao. Hemicyanine-Based Type I Photosensitizers for Antihypoxic Activatable Photodynamic Therapy. ACS Materials Lett. 2023, 5, 3058. https://doi.org/10.1021/acsmaterialslett.3c00933
(a) S. Liu, H. Zhang, Y. Li, J. Liu, L. Du, M. Chen, R. T. K. Kwok, J. W. Y. Lam, D. L. Phillips, B. Z. Tang. Strategies to Enhance the Photosensitization: Polymerization and the Donor–Acceptor Even–Odd Effect. Angew. Chem., Int. Ed. 2018, 57, 15189. https://doi.org/10.1002/anie.201810326 (b) W. Wu, D. Mao, F. Hu, S. Xu, C. Chen, C.-J. Zhang, X. Cheng, Y. Yuan, D. Ding, D. Kong, B. Liu. A Highly Efficient and Photostable Photosensitizer with Near-Infrared Aggregation-Induced Emission for Image-Guided Photodynamic Anticancer Therapy. Adv. Mater. 2017, 29, 1700548. https://doi.org/10.1002/adma.201700548 (c) M. Kang, C. Zhou, S. Wu, B. Yu, Z. Zhang, N. Song, M. M. S. Lee, W. Xu, F. J. Xu, D. Wang, L. Wang, B. Z. Tang. Evaluation of Structure–Function Relationships of Aggregation-Induced Emission Luminogens for Simultaneous Dual Applications of Specific Discrimination and Efficient Photodynamic Killing of Gram-Positive Bacteria. J. Am. Chem. Soc. 2019, 141, 16781. https://doi.org/10.1021/jacs.9b07162
Z. Zhuang, J. Dai, M. Yu, J. Li, P. Shen, R. Hu, X. Lou, Z. Zhao, B. Z. Tang. Type I photosensitizers based on phosphindole oxide for photodynamic therapy: apoptosis and autophagy induced by endoplasmic reticulum stress. Chem. Sci. 2020, 11, 3405. https://doi.org/10.1039/D0SC00785D
F. Hu, S. Xu, B. Liu. Photosensitizers with Aggregation-Induced Emission: Materials and Biomedical Applications. Adv. Mater. 2018, 30, 1801350. https://doi.org/10.1002/adma.201801350
Q. Wan, R. Zhang, Z. Zhuang, Y. Li, Y. Huang, Z. Wang, W. Zhang, J. Hou, B. Z. Tang. Molecular Engineering to Boost AIE-Active Free Radical Photogenerators and Enable High-Performance Photodynamic Therapy under Hypoxia. Adv. Funct. Mater. 2020, 30, 2002057. https://doi.org/10.1002/adfm.202002057
(a) P. Xiao, Z. Shen, D. Wang, Y. Pan, Y. Li, J. Gong, L. Wang, D. Wang, B. Z. Tang. Precise Molecular Engineering of Type I Photosensitizers with Near-Infrared Aggregation-Induced Emission for Image-Guided Photodynamic Killing of Multidrug-Resistant Bacteria. Adv. Sci. 2022, 9,104079. https://doi.org/10.1002/advs.202104079 (b) X. Zhao, Y. Dai, F. Ma, S. Misal, K. Hasrat, H. Zhu and Z. Qi. Molecular engineering to accelerate cancer cell discrimination and boost AIE-active type I photosensitizer for photodynamic therapy under hypoxia. Chem. Eng. J. 2021, 410, 128133. https://doi.org/10.1016/j.cej.2020.128133 (c) Z. Liu, Q. Wang, W. Qiu, Y. Lyu, Z. Zhu, X. Zhao, W. H. Zhu. AIE-active luminogens as highly efficient free-radical ROS photogenerator for image-guided photodynamic therapy. Chem. Sci. 2022, 13, 3599. https://doi.org/10.1039/D2SC00067A (d) K. Chen, P. He, Z. Wang, B. Z. Tang. A Feasible Strategy of Fabricating Type I Photosensitizer for Photodynamic Therapy in Cancer Cells and Pathogens. ACS Nano, 2021, 15, 7735. https://doi.org/10.1021/acsnano.1c01577
(a) X. Ma, Y. Zhao. Biomedical Applications of Supramolecular Systems Based on Host–Guest Interactions. Chem. Rev. 2015, 115, 7794. https://doi.org/10.1021/cr500392w (b) M. Li, Z. Luo, Y. Zhao. Self-Assembled Hybrid Nanostructures: Versatile Multifunctional Nanoplatforms for Cancer Diagnosis and
Therapy. Chem. Mater. 2018, 30, 25. https://doi.org/10.1021/acs.chemmater.7b03924
S. Liu, B. Wang, Y. Yu, Y. Liu, Z, Zhuang, Z, Zhao, G. Feng, A. Qin, B. Z. Tang. Cationization-Enhanced Type I and Type II ROS Generation for Photodynamic Treatment of Drug-Resistant Bacteria. ACS Nano 2022, 16, 9130. https://doi.org/10.1021/acsnano.2c01206
L. Feng, C. Li, L. Liu, Z. Wang, Z. Chen, J. Yu, W. Ji, G. Jiang, P. Zhang, J. Wang, B. Z. Tang. Acceptor Planarization and Donor Rotation: A Facile Strategy for Realizing Synergistic Cancer Phototherapy via Type I PDT and PTT. ACS Nano 2022, 16, 4162. https://doi.org/10.1021/acsnano.1c10019
J. Zhao, R. Huang, Y. Gao, J. Xu, Y. Sun, J. Bao, L. Fang, S. Gou. Realizing Near-Infrared (NIR)-Triggered Type-I PDT and PTT by Maximizing the Electronic Exchange Energy of Perylene Diimide-Based Photosensitizers, ACS Materials Lett. 2023, 5, 1752. https://doi.org/10.1021/acsmaterialslett.3c00436
M. M. S. Lee, D. M. Lin, J. H. C. Chau, E. Y. Yu, D. Ding, R. T. K. Kwok, D. Wang, B. Z. Tang. Adipocyte-Targeting Type I AIE Photosensitizer for Obesity Treatment via Photodynamic Lipid Peroxidation. ACS Nano 2023, 17, 11039. https://doi.org/10.1021/acsnano.3c03654
Z. Li, F. Ni, S. Jia, L.-H. Gao, H. Yuan, K.-Z. Wang. Bipolar Hemicyanine-Based Photodynamic Modulation of Type I Pathway for Efficient Sterilization and Real-Time Monitoring. ACS Appl. Bio Mater. 2022, 5, 2549. https://doi.org/10.1021/acsabm.2c00394
Y. Wang, Y. Sun, J. Ran, H. Yang, S. Xiao, J. Yang, C. Yang, H. Wang, Y. Liu. Utilization of Nonradiative Excited-State Dissipation for Promoted Phototheranostics Based on an AIE-Active Type I ROS Generator. ACS Appl. Mater. Interfaces 2022, 14, 225. https://doi.org/10.1021/acsami.1c19008
G. Yang, S.-B. Lu, C. Li, F. Chen, J.-S. Ni, M. Zha, Y. Li, J. Gao, T. Kang, C. Liu, K. Li. Type I macrophage activator photosensitizer against hypoxic tumors. Chem. Sci. 2021, 12, 14773. https://doi.org/10.1039/D1SC04124J
S. Zhou, R. Li, Y. Li, Y. Wang, L. Feng. A tailored and red-emissive type I photosensitizer to potentiate photodynamic immunotherapy, J. Mater. Chem. B 2022, 10, 8003. https://doi.org/10.1039/D2TB01578A
H. Huang, S. Long, D. Huang, J. Du, J. Fan and X. Peng. A photosensitizer with conformational restriction for enhanced photodynamic therapy. Chem. Commun. 2021, 57, 9100. https://doi.org/10.1039/D1CC03591F
Y. Wang, J. Li, Y. Zhang, Y. Nan, X. Zhou. Rational design of a meso phosphate-substituted pyronin as a type I photosensitizer for photodynamic therapy. Chem. Commun. 2022, 58, 7797. https://doi.org/10.1039/D2CC02124B
L. Zhao, Y. Xing, R. Wang, F. Yu, F. Yu. Self-Assembled Nanomaterials for Enhanced Phototherapy of Cancer. ACS Appl. Bio Mater. 2020, 3, 86. https://doi.org/10.1021/acsabm.9b00843
J. Cheng, H. Zhao, L. Yao, Y. Li, B. Qi, J. Wang, X. Yang. Simple and Multifunctional Natural Self-Assembled Sterols with Anticancer Activity-Mediated Supramolecular Photosensitizers for Enhanced Antitumor Photodynamic Therapy. ACS Appl. Mater. Interfaces 2019, 11, 29498. https://doi.org/10.1021/acsami.9b07404
S. Chao, Z. Shen, B. Li, Y. Pei, Z. Pei. An L-arginine-functionalized pillar[5]arene-based supramolecular photosensitizer for synergistically enhanced cancer therapeutic effectiveness.
Chem. Commun. 2023, 59, 3455. https://doi.org/10.1039/D3CC00123G
(a) K. X. Teng, L. Y. Niu and Q. Z. Yang. A host–guest strategy for converting the photodynamic agents from a singlet oxygen generator to a superoxide radical generator. Chem. Sci. 2022, 13, 5951. https://doi.org/10.1039/D2SC01469F (b) K. X. Teng, L. Y. Niu, N. Xie and Q. Z. Yang. Supramolecular photodynamic agents for simultaneous oxidation of NADH and generation of superoxide radical. Nat. Commun. 2022, 13, 6179. https://doi.org/10.1038/s41467-022-33924-3
(a) H. Shigemitsu, Y. Tani, T. Tamemoto, T. Mori, X. Li, Y. Osakada, M. Fujitsuka, T. Kida. Aggregation-induced photocatalytic activity and efficient photocatalytic hydrogen evolution of
amphiphilic rhodamines in water. Chem. Sci. 2020, 11, 11843. https://doi.org/10.1039/D0SC04285D (b) H. Shigemitsu, K. Ohkubo, K. Sato, A. Bunno, T. Mori, Y. Osakada, M. Fujitsuka, T. Kida. Fluorescein-Based Type I Supramolecular Photosensitizer via Induction of Charge Separation by Self-Assembly. JACS Au 2022, 2, 1472. https://doi.org/10.1021/jacsau.2c00243
H. Shigemitsu, K. Sato, S. Hagio, Y. Tani, T. Mori, K. Ohkubo, Y. Osakada, M. Fujitsuka, T. Kida. Amphiphilic Rhodamine Nano-assembly as a Type I Supramolecular Photosensitizer for Photodynamic Therapy. ACS Appl. Nano Mater. 2022, 5, 14954. https://doi.org/10.1021/acsanm.2c03192