Water-Soluble Biocompatible Polymers from Sustainable Amino Acid Moieties for Biomedical Applications Polymer Science
Main Article Content
Abstract
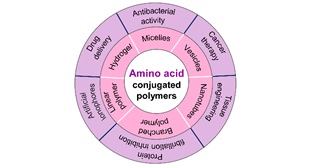
Amino acids have emerged as sustainable and versatile building blocks for the creation of functional polymers. Harnessing their intrinsic structural diversity and biological relevance enables the design of unique, well-defined, biocompatible, and highly tailorable macromolecules. Beyond their natural abundance, the exceptional biocompatibility of amino acid-derived polymers positions them as promising candidates for a wide spectrum of biomedical applications. Moreover, the diverse reactive functionalities of amino acids (such as carboxylic acid, amine, thiol, and hydroxyl groups) provide a powerful synthetic toolbox, supporting a broad range of polymerization strategies from step-growth to chain-growth mechanisms. This review highlights the recent developments (2015-present) in the synthesis of amino acid-conjugated polymeric architectures and their biomedical applications. By bridging molecular design, polymer chemistry, and bio-functionality, we highlight the transformative potential of these bioinspired materials and anticipate their pivotal role in shaping the next generation of sustainable and therapeutic polymer platforms.
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.
How to Cite
References
J. Zhu, N. Avakyan, A. Kakkis, A. M. Hoffnagle, K. Han, Y. Li, Z. Zhang, T. S. Choi, Y. Na, C. -J. Yu, F. A. Tezcan. Protein assembly by design. Chem. Rev. 2021, 121, 13701. https://doi.org/10.1021/acs.chemrev.1c00308
A. Levin, T. A. Hakala, L. Schnaider, G. J. L. Bernardes, E. Gazit, T. P. J. Knowles. Biomimetic peptide self-assembly for functional materials. Nat. Rev. Chem. 2020, 4, 615. https://doi.org/10.1038/s41570-020-0215-y
Q. Luo, C. Hou, Y. Bai, R. Wang, J. Liu. Protein assembly: versatile approaches to construct highly ordered nanostructures. Chem. Rev. 2016, 116, 13571. https://doi.org/10.1021/acs.chemrev.6b00228
Y. Wang, S. Rencus-Lazar, H. Zhou, Y. Yin, X. Jiang, K. Cai, E. Gazit, W. Ji. Bioinspired amino acid based materials in bionanotechnology: from minimalistic building blocks and assembly mechanism to applications. ACS Nano 2024, 18, 1257. https://doi.org/10.1021/acsnano.3c08183
S. S. Gupta, V. Mishra, M. D. Mukherjee, P. Saini, K. R. Ranjan. Amino acid derived biopolymers: recent advances and biomedical applications. Int. J. Biol. Macromol. 2021, 188, 542. https://doi.org/10.1016/j.ijbiomac.2021.08.036
H. Sun, F. Meng, A. A. Dias, M. Hendriks, J. Feijen, Z. Zhong. α-Amino acid containing degradable polymers as functional biomaterials: rational design, synthetic pathway, and biomedical applications. Biomacromolecules 2011, 12, 1937. https://doi.org/10.1021/bm200043u
K. Tao, A. Levin, L. Adler-Abramovich, E. Gazit. Fmoc-modified amino acids and short peptides: simple bio-inspired building blocks for the fabrication of functional materials. Chem. Soc. Rev. 2016, 45, 3935. https://doi.org/10.1039/C5CS00889A
M. N. Leiske, K. Kempe. A guideline for the synthesis of amino-acid-functionalized monomers and their polymerizations. Macromol. Rapid Commun. 2022, 43, 2100615. https://doi.org/10.1002/marc.202100615
H. R. Kricheldorf. Polypeptides and 100 years of chemistry of α-amino acid N-carboxyanhydrides. Angew. Chem., Int. Ed. 2006, 45, 5752. https://doi.org/10.1002/anie.200600693
N. Ozawa, T. Nishimura. Macromolecular architectural effects on solution self-assembly of amphiphilic AB-type block copolymers. Polym. Chem. 2024, 15, 349. https://doi.org/10.1039/D3PY01324C
Y. Han, Z. Chen, J. Wang, Y. Jin, H. Ma, Z. Yang, Y. Zhang, M. Ma, Z. Lei, and D. Lu. Targeted embolization and chemotherapy for solid tumors by microenvironment-responsive poly(amino acid)s. J. Macromol. Sci., Part A: Pure Appl. Chem. 2023, 61, 79. https://doi.org/10.1080/10601325.2023.2296632
F. Sanda, T. Endo. Syntheses and functions of polymers based on amino acids. Macromol. Chem. Phys. 1999, 200, 2651. https://doi.org/10.1002/(SICI)15213935(19991201)200:12%3C2651::AID-MACP2651%3E3.0.CO;2-P
K. Nayak, P. Ghosh, M. E. H. Khan, P. De. Side‐chain amino‐acid‐based polymers: self‐assembly and bioapplications. Polym. Int. 2022, 71, 411. https://doi.org/10.1002/pi.6278
A. Mondal, S. Das, A. Banerjee, A. Sengupta, M. R. Molla. Amphiphilic poly(disulfide)s by a three-component cascade reactions: a therapeutic architecture for redox-responsive intracellular drug delivery. J. Macromol. Sci., Part A: Pure Appl. Chem. 2025, 62, 212. https://doi.org/10.1080/10601325.2025.2454283
X. -Z. Wang, J. -S. Yao. Effect of amino acid pendant groups on the helical configuration of polyphenylacetylene. J. Macromol. Sci. Part A: Pure Appl. Chem. 2023, 60, 409. https://doi.org/10.1080/10601325.2023.2211613
H. A. Zayas, A. Lu, D. Valade, F. Amir, Z. Jia, R. K. O'Reilly, M. J. Monteiro. Thermoresponsive polymer-supported L-proline micelle catalysts for the direct asymmetric aldol reaction in water. ACS Macro Lett. 2013, 2, 327. https://doi.org/10.1021/mz4000943
C. Deng, J. Wu, R. Cheng, F. Meng, H. -A. Klok, Z. Zhong. Functional polypeptide and hybrid materials: precision synthesis via α-amino acid N-carboxyanhydride polymerization and emerging biomedical applications. Prog. Polym. Sci. 2014, 39, 330. https://doi.org/10.1016/j.progpolymsci.2013.10.008
S. Kumar, R. Acharya, U. Chatterji, P. De. Controlled synthesis of pH responsive cationic polymers containing side-chain peptide moieties via raft polymerization and their self-assembly. J. Mater. Chem. B Mater. Biol. Med. 2013, 1, 946. https://doi.org/10.1039/c2tb00170e
P. Chakraborty, E. Gazit. Amino acid based self-assembled nanostructures: complex structures from remarkably simple building blocks. ChemNanoMat 2018, 4, 730. https://doi.org/10.1002/cnma.201800147
H. Yuan, M. Jiang, H. Fang, H. Tian. Recent advances in poly(amino acids), polypeptides, and their derivatives in drug delivery. Nanoscale 2025, 17, 3549. https://doi.org/10.1039/d4nr04481a
M. P. Hendricks, K. Sato, L. C. Palmer, S. I. Stupp. Supramolecular assembly of peptide amphiphiles. Acc. Chem. Res. 2017, 50, 2440. https://doi.org/10.1021/acs.accounts.7b00297
G. Wei, Z. Su, N. P. Reynolds, P. Arosio, I. W. Hamley, E. Gazit, R. Mezzenga. Self-assembling peptide and protein amyloids: from structure to tailored function in nanotechnology. Chem. Soc. Rev. 2017, 46, 4661. https://doi.org/10.1039/c6cs00542j
D. Kim, S. A. Han, J. H. Kim, J. -H. Lee, S. -W. Kim, S. -W. Lee. Biomolecular piezoelectric materials: from amino acids to living tissues. Adv. Mater. 2020, 32, 1906989. https://doi.org/10.1002/adma.201906989
W. Shen, P. He, C. Xiao, X. Chen. From antimicrobial peptides to antimicrobial poly(α-amino acid)s. Adv. Healthc. Mater. 2018, 7, 1800354. https://doi.org/10.1002/adhm.201800354
J. De Breuck, M. Streiber, M. Ringleb, D. Schröder, N. Herzog, U. S. Schubert, S. Zechel, A. Traeger, M. N. Leiske. Amino-acid-derived anionic polyacrylamides with tailored hydrophobicity-physicochemical properties and cellular interactions. ACS Polym. Au 2024, 4, 222. https://doi.org/10.1021/acspolymersau.3c00048
C. Xu, X. Hu, J. Wang, Y. -M. Zhang, X. -J. Liu, B. -B. Xie, C. Yao, Y. Li, X. -S. Li. Library of antifouling surfaces derived from natural amino acids by click reaction. ACS Appl. Mater. Interfaces 2015, 7, 17337. https://doi.org/10.1021/acsami.5b04520
X. Xu, Y. Li, H. Li, R. Liu, M. Sheng, B. He, Z. Gu. Smart nanovehicles based on pH-triggered disassembly of supramolecular peptide-amphiphiles for efficient intracellular drug delivery. Small 2014, 10, 1133. https://doi.org/10.1002/smll.201301885
X. Yang, B. Wang, D. Sha, Y. Liu, J. Xu, K. Shi, C. Yu, X. Ji. Injectable and antibacterial ε-poly(L-lysine)-modified poly(vinyl alcohol)/chitosan/AgNPs hydrogels as wound healing dressings. Polymer (Guildf.) 2021, 212, 123155. https://doi.org/10.1016/j.polymer.2020.123155
J. L. Nichol, N. L. Morozowich, H. R. Allcock. Biodegradable alanine and phenylalanine alkyl ester polyphosphazenes as potential ligament and tendon tissue scaffolds. Polym. Chem. 2013, 4, 600. https://doi.org/10.1039/c2py20631e
R. K. O’Reilly. Using controlled radical polymerisation techniques for the synthesis of functional polymers containing amino acid moieties. Polym. Int. 2010, 59, 568. https://doi.org/10.1002/pi.2830
H. Mori, T. Endo. Amino-acid-based block copolymers by RAFT polymerization. Macromol. Rapid Commun. 2012, 33, 1090. https://doi.org/10.1002/marc.201100887
S. G. Roy, P. De. pH responsive polymers with amino acids in the side chains and their potential applications. J. Appl. Polym. Sci. 2014, 131, 41084. https://doi.org/10.1002/app.41084
T. Maity, S. Paul, P. De. Side-chain amino acid-based macromolecular architectures. J. Macromol. Sci., Part A: Pure Appl. Chem. 2023, 60, 2. https://doi.org/10.1080/10601325.2023.2169158
D. Kumar, B. Sahu, S. Banerjee. Amino acid‐derived smart and functional polymers for biomedical applications: current status and future perspectives. Macromol. Chem. Phys. 2023, 224, 2300207. https://doi.org/10.1002/macp.202300207
U. A. Gavhane, D. C. Joshi, M. Jayakannan. Size- and shape-controlled biodegradable polymer brushes based on L-amino acid for intracellular drug delivery and deep-tissue penetration. Biomacromolecules 2024, 25, 3756. https://doi.org/10.1021/acs.biomac.4c00341
R. J. Pounder, A. P. Dove. Towards poly(ester) nanoparticles: recent advances in the synthesis of functional poly(ester)s by ring-opening polymerization. Polym. Chem. 2010, 1, 260. https://doi.org/10.1039/B9PY00327D
F. Sheehan, D. Sementa, A. Jain, M. Kumar, M. Tayarani-Najjaran, D. Kroiss, R. V. Ulijn. Peptide-based supramolecular systems chemistry. Chem. Rev. 2021, 121, 13869. https://doi.org/10.1021/acs.chemrev.1c00089
R. K. Kulkarni, H. Morawetz. Effect of asymmetric centers on free radical polymerization and the properties of polymers: methacrylyl alanine, methacrylyl glutamic acid, acrylyl glutamic acid, and their polymers. J. Polym. Sci. 1961, 54, 491. https://doi.org/10.1002/pol.1961.1205416014
H. Sun, C. Gao. Facile synthesis of multiamino vinyl poly(amino acid)s for promising bioapplications. Biomacromolecules 2010, 11, 3609. https://doi.org/10.1021/bm101060m
J. Nicolas, G. Mantovani, D. M. Haddleton. Living radical polymerization as a tool for the synthesis of polymer‐protein/peptide bioconjugates. Macromol. Rapid Commun. 2007, 28, 1083. https://doi.org/10.1002/marc.200700112
Y. Teng, S. Wu, X. Chen, L. Bai, Q. Wu, G. Zhang. pH and temperature dual-responsive magnetic yolk-shell mesoporous silicananoparticles for controlled drug delivery. J. Macromol. Sci., Part A: Pure Appl. Chem. 2024, 61, 640. https://doi.org/10.1080/10601325.2024.2380831
A. Okamura, T. Hirai, M. Tanihara, T. Yamaoka. Synthesis and properties of novel biodegradable polyamides containing α-amino acids. Polymer 2002, 12, 3549. https://doi.org/10.1016/s0032-3861(02)00111-8
A. Dey, U. Haldar, R. Tota, R. Faust, P. De. PIB-Based block copolymer with a segment having alternating sequence of leucine and alanine side-chain pendants. J. Macromol. Sci., Part A: Pure Appl. Chem. 2023, 60, 161. https://doi.org/10.1080/10601325.2023.2189434
Y. An, R. Zhai, J. Chen, P. Xie. Preparation and application of a novel pH-responsive linalool carboxymethyl chitosan hydrogel. J. Macromol. Sci., Part A: Pure Appl. Chem. 2023, 60, 336. https://doi.org/10.1080/10601325.2023.2195879
T. Maity, S. Paul, P. De. Designing functionalized polymers through post-polymerization modification of side chain leucine-based polymer. Eur. Polym. J. 2023, 199, 112445. https://doi.org/10.1016/j.eurpolymj.2023.112445
K. Manna, A. Roy, S. Dey, S. Ghosh, P. Pradhan, S. Pal. A review on the culmination of rational development of stimuli-responsive polymeric micelles as vehicles for site-specific hydrophobic therapeutics. J. Macromol. Sci. Part A: Pure Appl. Chem., 2024, 61, 349. https://doi.org/10.1080/10601325.2024.2335277
E. C¸akmakc¸ı. Recent advances in flame retardant polymers via thiol-ene click chemistry. J. Macromol. Sci., Part A: Pure Appl. Chem. 2023, 60, 817. https://doi.org/10.1080/10601325.2023.2280237
S. Biswas, P. Dey, G. Ghosh. Polymeric microgels: Synthesis, and emerging biomedical applications. J. Macromol. Sci., Part A: Pure Appl. Chem. 2025, 62, 295. https://doi.org/10.1080/10601325.2025.2471852
S. Zhu, R. Xue, Z. Yu, X. Zhang, S. Luan, H. Tang. Transition of conformation and solubility in β-sheet-structured poly(L-cysteine)s with methylthio or sulfonium pendants. Biomacromolecules 2021, 22, 1211. https://doi.org/10.1021/acs.biomac.0c01715
M. Xiong, Z. Han, Z. Song, J. Yu, H. Ying, L. Yin, J. Cheng. Bacteria-assisted activation of antimicrobial polypeptides by a random-coil to helix transition. Angew. Chem., Int. Ed. 2017, 56, 10826. https://doi.org/10.1002/anie.201706071
D. Li, S. Shi, D. Zhao, Y. Rong, Y. Zhou, J. Ding, C. He, X. Chen. Effect of polymer topology and residue chirality on biodegradability of polypeptide hydrogels. ACS Biomater. Sci. Eng. 2022, 8, 626. https://doi.org/10.1021/acsbiomaterials.1c01127
I. Mukherjee, A. Ghosh, P. Bhadury, P. De. Side-chain amino acid-based cationic antibacterial polymers: investigating the morphological switching of a polymer-treated bacterial cell. ACS omega 2017, 2, 1633. https://pubs.acs.org/doi/10.1021/acsomega.7b00181
A. Banerjee, A. Ghosh, B. Saha, P. Bhadury, P. De. Surface charge-switchable antifouling block copolymer with bacteriostatic properties. Langmuir 2024, 40, 5314. https://doi.org/10.1021/acs.langmuir.3c03771
D. Ghosh, S. Yadav, S. Bag, A. I. Mallick, P. De. Antibacterial activity of hydrophobicity modulated cationic polymers with enzyme and pH-responsiveness. J. Mater. Chem. B 2024, 12, 2894. https://doi.org/10.1039/d3tb02801a
S. Kumar, R. Acharya, U. Chatterji, P. De. Side-chain amino-acid-based pH-responsive self-assembled block copolymers for drug delivery and gene transfer. Langmuir 2013, 29, 15375. https://pubs.acs.org/doi/full/10.1021/la403819g
H. Li, T. Luo, R. Sheng, J. Sun, Z. Wang, A. Cao. Achieving high gene delivery performance with caveolae-mediated endocytosis pathway by (L)-arginine/(L)-histidine co-modified cationic gene carriers. Colloids Surf. B Biointerfaces 2016, 148, 73. https://doi.org/10.1016/j.colsurfb.2016.08.035
N. Liu, J. Han, X. Zhang, Y. Yang, Y. Liu, Y. Wang, G. Wu. pH-responsive zwitterionic polypeptide as a platform for anti-tumor drug delivery. Colloids Surf. B Biointerfaces 2016, 145, 401. https://doi.org/10.1016/j.colsurfb.2016.05.027
R. Kanto, Y. Qiao, K. Masuko, H. Furusawa, S. Yano, K. Nakabayashi, H. Mori. Synthesis, assembled structures, and DNA complexation of thermoresponsive lysine-based zwitterionic and cationic block copolymers. Langmuir 2019, 35, 4646. http://dx.doi.org/10.1021/acs.langmuir.8b04303
L. P. Datta, D. De, U. Ghosh, T. K. Das. RAFT derived fatty acid based stimuli responsive fluorescent block copolymers as DNA sensor and cargo delivery agent. Polymer 2018, 138, 103. https://doi.org/10.1016/j.polymer.2018.01.044
P. Ghosh, A. Bera, A. Ghosh, P. Bhadury, P. De. Side-chain proline-based polymers as effective inhibitors for in vitro aggregation of insulin. ACS Appl. Bio Mater. 2020, 3, 5407. https://doi.org/10.1021/acsabm.0c00709
A. Bera, P. Ghosh, K. Goswami, P. De. Amino acid-based polymer-coated silver nanoparticles as insulin fibril inhibitors. ACS Appl. Nano Mater. 2023, 6, 8705. https://doi.org/10.1021/acsanm.3c01078
K. Nayak, P. Ghosh, S. Barman, B. Sudhamalla, P. Theato, P. De. Amyloid β-peptide segment conjugated side-chain proline-based polymers as potent inhibitors in lysozyme amyloidosis. Bioconjugate Chem. 2024, 35, 312. https://doi.org/10.1021/acs.bioconjchem.3c00509
B. Maiti, P. Dutta, S. Seal, S. Pal, P. De, S. Maiti. Side-chain amino acid based cationic polymer induced actin polymerization. J. Mater. Chem. B 2017, 5, 1218. https://doi.org/10.1039/c6tb02814d
S. Sahoo, I. Maiti, A. Laha, R. De, S. Maiti, P. De. Cholate-conjugated cationic polymers for regulation of actin dynamics. J. Mater. Chem. B 2022, 10, 8033. https://doi.org/10.1039/d2tb01364a
G. H. Park, M. - S. Kang, J. C. Knowles, M. -S. Gong. Synthesis, characterization, and biocompatible properties of alanine-grafted chitosan copolymers. J. Biomater. Appl. 2016, 30, 1350. https://doi.org/10.1177/0885328215626892
J. Zhu, H. Han, F. Li, X. Wang, J. Yu, X. Qin, D. Wu. Peptide-functionalized amino acid-derived pseudoprotein-based hydrogel with hemorrhage control and antibacterial activity for wound healing. Chem. Mater. 2019, 31, 4436. https://doi.org/10.1021/acs.chemmater.9b00850
U. Haldar, K. Bauri, R. Li, R. Faust, P. De. Polyisobutylene-based pH-responsive self-healing polymeric gels. ACS Appl. Mater. Interfaces 2015, 7, 8779. https://doi.org/10.1021/acsami.5b01272
B. Saha, K. Bauri, A. Bag, P. K. Ghorai, P. De. Conventional fluorophore-free dual pH- and thermo-responsive luminescent alternating copolymer. Polym. Chem. 2016, 7, 6895. https://doi.org/10.1039/c6py01738j
K. Bauri, B. Saha, J. Mahanti, P. De. A nonconjugated macromolecular luminogen for speedy, selective and sensitive detection of picric acid in water. Polym. Chem. 2017, 8, 7180. https://doi.org/10.1039/c7py01579h
N. Choudhury, B. Ruidas, C. Das Mukhopadhyay, P. De. Rhodamine-appended polymeric probe: an efficient colorimetric and fluorometric sensing platform for Hg2+ in aqueous medium and living cells. ACS Appl. Polym. Mater. 2020, 2, 5077. https://doi.org/10.1021/acsapm.0c00878
N. Choudhury, B. Ruidas, B. Saha, K. Srikanth, C. Das Mukhopadhyay, P. De. Multifunctional tryptophan-based fluorescent polymeric probes for sensing, bioimaging and removal of Cu2+ and Hg2+ ions. Polym. Chem. 2020, 11, 2015. https://doi.org/10.1039/c9py01892a
S. Roy, S. Pan, N. Choudhury, S. Sivaram, P. De. Water-soluble polymeric probe with tryptophan pendants for formaldehyde sensing. Eur. Polym. J. 2024, 215, 113241. https://doi.org/10.1016/j.eurpolymj.2024.113241
A. Mondal, A. Pal, S. Sarkar, R. Datta, P. De. Antioxidant polymers with phenolic pendants for the mitigation of cellular oxidative stress. Biomacromolecules 2024, 25, 1649. https://doi.org/10.1021/acs.biomac.3c01193
A. Dey, U. Haldar, T. Rajasekhar, R. Faust, P. De. Charge variable PIB-based block copolymers as selective transmembrane ion transporters. Polym. Chem. 2023, 14, 2581. https://doi.org/10.1039/d3py00274h
A. Lu, T. P. Smart, T. H. III. Epps, D. A. Longbottom, R. K. O’Reilly. L-Proline functionalized polymers prepared by RAFT polymerization and their assemblies as supported organocatalysts. Macromolecules 2011, 44, 7233. https://doi.org/10.1021/ma201256m
T. Mallick, D. Jana, A. Bisai, P. De. Asymmetric aldol reactions catalyzed by polymeric self-assembly with side-chain dipeptide pendants. ACS Macro Lett. 2024, 13, 651. https://doi.org/10.1021/acsmacrolett.4c00185
P. Chowdhury, B. Saha, K. Bauri, B. S. Sumerlin, P. De. Hydrogen bonding-driven self-coacervation of nonionic homopolymers for stimuli-triggered therapeutic release. J. Am. Chem. Soc. 2024, 146, 21664. https://doi.org/10.1021/jacs.4c05624