Eco-Friendly Sustainable Vaccine Designing Technology Aiding Personalised Immunotherapy Medicinal Chemistry
Main Article Content
Abstract
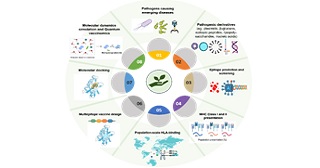
With the rise of pandemic and endemic cases in recent years, vaccination has been prioritised along with prophylactic therapies. The development of a vaccine candidate sometimes requires lengthy timelines of research and resources. Apart from the unpredictable side effects experienced by individuals, most of the vaccination efforts suffer from population coverage and serotype specificity. Also, lots of euthanised mammals are required for evaluating lethal doses and pharmacokinetic parameters. Worldwide genetic diversity for various human leukocyte antigens (HLAs) or alleles is one of the reasons for zone-specific clinical trials. In these cases, rigorous studies with healthy subjects are required to report statistically reasonable data. In the era of reverse vaccinology, it is now possible to predict immunoproteasomal cleavages of an antigen (upon cellular uptake) and possible nonamers produced from it. These nonamers can be identified for their allergenic, pro-inflammatory characteristics utilising machine learning (ML) and deep learning (DL) algorithms, integrating AI (Artificial Neural Network) driven epitope prediction with HLA-specific binding analyses. These computational tools reduce reliance on animal trials by simulating epitope interactions in-silico, addressing sustainable goals in peptide vaccine development for global populations. This review aims to summarise a computational workflow that can lead to an antigenic peptide as a protective immunogen in individuals.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
How to Cite
References
A. Bouazzaoui, A. A. H. Abdellatif, F. A. Al-Allaf, N. M. Bogari, S. Al-Dehlawi, S. H. Hari. Strategies for Vaccination: Conventional Vaccine Approaches Versus New-Generation Strategies in Combination with Adjuvants. Pharmaceutics, 2021, 13, 140. https://doi.org/10.3390/pharmaceutics13020140
S. Pattanayak. LIMITATIONS OF THE CONTEMPORARY VACCINES: HOW TO OVERCOME? Explor. Anim. Med. Res., 2023, 13, 140.
A. Detmer, J. Glenting. Live bacterial vaccines a review and identification of potential hazards. Microb. Cell Fact., 2006, 5, 23. https://doi.org/10.1186/1475-2859-5-23
R. Verbeke, M. H. Y. Cheng, P. R. Cullis. A Historical Overview on mRNA Vaccine Development. In: Trends in mRNA Vaccine Research, 2025, 1. https://doi.org/10.1002/9783527838394.ch1
F. Zahedipour, K. Jamialahmadi, P. Zamani, M. R. Jaafari. Improving the efficacy of peptide vaccines in cancer immunotherapy. Int. Immunopharmacol., 2023, 123. https://doi.org/10.1016/j.intimp.2023.110721
P. Sadhukhan, I. Sutnga, B. Bingari, A. Choudhury. A Retrospective Assessment of the Initial Phase of Covid-19 and Its Implemented Treatment Strategies. J. Drug Deliv. Ther., 2021, 11, 81. https://doi.org/10.22270/jddt.v11i3-S.4868
A. Patel, R. Patel. Next-Generation Vaccine Development: mRNA, Viral Vector, and Protein-Based Approaches for Pandemic Preparedness. Int. J. Innov. Res. Sci. Eng. Technol., 2024, 13, 15577. https://doi.org/10.15680/IJIRSET.2024.1308180
N. Z. Miller, G. S. Goldman. Neonatal, Infant, and Under Age Five Vaccine Doses Routinely Given in Developed Nations and Their Association With Mortality Rates. Cureus, 2023, 15, e42194. https://doi.org/10.7759/cureus.42194
DISEASES COI, Y. A. Maldonado, S. T. O’Leary, R. Banerjee, J. D. Campbell, M. T. Caserta, J. S. Gerber, A. P. Kourtis, R. Lynfield, F. M. Munoz, D. Nolt, A. Ratner, S. S. Shah, W. J. Steinbach, K. M. Zangwill, T. E. Zaoutis. Recommended Childhood and Adolescent Immunization Schedule: United States, 2021. Pediatrics, 2021, 147, e2020049775. https://doi.org/10.1542/peds.2020-049775
R. Hutubessy, J. A. Lauer, B. Giersing, S. Y. Sim, M. Jit, D. Kaslow & S. Botwright. The Full Value of Vaccine Assessments (FVVA): a framework for assessing and communicating the value of vaccines for investment and introduction decision-making. BMC medicine, 2023, 21. https://doi.org/10.1186/s12916-023-02929-0
W. P. Hausdorff, J. D. Anderson IV, A. L. Bourgeois, A. Clifford, J. A. Fleming, F. Muhib, C. Pecenka, C. Puett, M. S. Riddle, S. Scheelea & K. H. Bagamian. Reassessing potential economic value and health impact of effective Shigella vaccines. Bull. World Health Organ., 2024, 102, 65. https://doi.org/10.2471/blt.23.290163
B. Giersing, A. X. Mo, A. Hwang, S. Baqar, K. Earle, A. Ford, C. Deal, P. Dull, M. Friede, B. Fenton Hall. Meeting summary: Global vaccine and immunization research forum, 2023. Vaccine, 2025, 46. https://doi.org/10.1016/j.vaccine.2024.126686
A. Alberts, S. K. Kjaer, S. H. Søegaard, J. F. Winther, K. Schmiegelow, L. Sopina, F. Erdmann, M. Hargreave. Childhood vaccinations and the risk of leukemia: A nationwide cohort study. Int. J. Cancer., 2025, 157, 55. https://doi.org/10.1002/ijc.35338
A. C. MacArthur, M. L. McBride, J. J. Spinelli, S. Tamaro, R. P. Gallagher, G. P. Theriault. Risk of Childhood Leukemia Associated with Vaccination, Infection, and Medication Use in Childhood: The Cross-Canada Childhood Leukemia Study. Am. J. Epidemiol., 2007, 167, 598. https://doi.org/10.1093/aje/kwm339
M. Marron, L. K. Brackmann, P. Kuhse, L. Christianson, I. Langner, U. Haug, W. Ahrens. Vaccination and the Risk of Childhood Cancer-A Systematic Review and Meta-Analysis. Front. Oncol., 2020, 10. https://doi.org/10.3389/fonc.2020.610843
D. Amodio, A. Angelidou, N. Cotugno, A. C. Sherman, O. Levy, P. Palma. Biomarkers of vaccine safety and efficacy in vulnerable populations: Lessons from the fourth international precision vaccines conference. Vaccine, 2025, 43. https://doi.org/10.1016/j.vaccine.2024.126477
Y. Li, J. Li, J. He, C Tao. AE-GPT: Using Large Language Models to extract adverse events from surveillance reports-A use case with influenza vaccine adverse events. PLoS One, 2024, 19. https://doi.org/10.1371/journal.pone.0300919
I. A. Doytchinova, D. R. Flower. VaxiJen: a server for prediction of protective antigens, tumour antigens and subunit vaccines. BMC Bioinformatics, 2007, 8. https://doi.org/10.1186/1471-2105-8-4
C. N. Magnan, M. Zeller, M. A. Kayala, A. Vigil, A. Randall, P. L. Felgner, P. Baldi. High-throughput prediction of protein antigenicity using protein microarray data. Bioinformatics, 2010, 26, 2936. https://doi.org/10.1093/bioinformatics/btq551
I. Dimitrov, I. Bangov, D. R. Flower, I. Doytchinova. AllerTOP v.2—a server for in silico prediction of allergens. J. Mol. Model., 2014, 20. https://doi.org/10.1007/s00894-014-2278-5
S. Saha, G. P. S. Raghava. AlgPred: prediction of allergenic proteins and mapping of IgE epitopes. Nucleic Acids Res., 2006, 34, W202. https://doi.org/10.1093/nar/gkl343
M. N. Nguyen, N. L. Krutz, V. Limviphuvadh, A. L. Lopata, G. F. Gerberick, S. Maurer-Stroh. AllerCatPro 2.0: a web server for predicting protein allergenicity potential. Nucleic Acids Res., 2022, 50, W36. https://doi.org/10.1093/nar/gkac446
A. S. Rathore, S. Choudhury, A. Arora, P. Tijare, G. P.S. Raghava. ToxinPred 3.0: An improved method for predicting the toxicity of peptides. Comput. Biol. Med., 2024, 179. https://doi.org/10.1016/j.compbiomed.2024.108926
Q. Yu, Z. Zhang, G. Liu, W. Li, Y. Tang. ToxGIN: an In silico prediction model for peptide toxicity via graph isomorphism networks integrating peptide sequence and structure information. Brief. Bioinform., 2024, 25. https://doi.org/10.1093/bib/bbae583
M. Wang, L. Kurgan, M. Li. Comprehensive assessment and comparison of tools for HLA class I peptide-binding prediction. Brief. Bioinform., 2023, 24. https://doi.org/10.1093/bib/bbad150
R. Vita, N. Blazeska, D. Marrama, IEDB Curation Team Members, S. Duesing, J. Bennett, J. Greenbaum, M. D. A. Mendes, J. Mahita, D. K. Wheeler, J. R. Cantrell, J. A. Overton, D. A. Natale, A. Sette, B. Peters. The Immune Epitope Database (IEDB): 2024 update. Nucleic Acids Res., 2025, 53, D436. https://doi.org/10.1093/nar/gkae1092
J. B. Nilsson, A. Grifoni, A. Tarke, A. Sette, M. Nielsen. PopCover-2.0. Improved Selection of Peptide Sets With Optimal HLA and Pathogen Diversity Coverage. Front. Immunol., 2021, 12. https://doi.org/10.3389/fimmu.2021.728936
H. H. Bui, J. Sidney, K. Dinh, S. Southwood, M. J. Newman, A. Sette. Predicting population coverage of T-cell epitope-based diagnostics and vaccines. BMC Bioinformatics, 2006, 7. https://doi.org/10.1186/1471-2105-7-153
M. S. B. Islam, M. Miah, M. E Hossain, K. M. K. Kibria. A conserved multi-epitope-based vaccine designed by targeting hemagglutinin protein of highly pathogenic avian H5 influenza viruses. 3 Biotech., 2020, 10. https://doi.org/10.1007/s13205-020-02544-3
B. E. Haug, K. A. Camilio, L. T. Eliassen, W. Stensen, J. S. Svendsen, K. Berg, B. Mortensen, G. Serin, J. F. Mirjolet, F. Bichat, Ø. Rekdal. Discovery of a 9-mer Cationic Peptide (LTX-315) as a Potential First in Class Oncolytic Peptide. J. Med. Chem., 2016, 59, 2918. https://doi.org/10.1021/acs.jmedchem.5b02025
H. Zhang, L. Liu, J. Zhang, J. Chen, J. Ye, S. Shukla, J. Qiao, X. Zhan, H. Chen, C. J. Wu, Y. X. Fu, Bo Li. Investigation of Antigen-Specific T-Cell Receptor Clusters in Human Cancers. Clin. Cancer. Res., 2019, 26. https://doi.org/10.1158/1078-0432.CCR-19-3249
G. S. Antipas, A. E. Germenis. Atomic Coordination Reflects Peptide Immunogenicity. Front. Mol. Biosci., 2015, 2. https://doi.org/10.3389/fmolb.2015.00077
W. C. Lim, A. M. Khan. Mapping HLA-A2, -A3 and -B7 supertype-restricted T-cell epitopes in the ebolavirus proteome. BMC Genomics, 2018, 19. https://doi.org/10.1186/s12864-017-4328-8
B. Bacsa, K. Horváti, S. Bõsze, F. Andreae, C. O. Kappe. Solid-Phase Synthesis of Difficult Peptide Sequences at Elevated Temperatures: A Critical Comparison of Microwave and Conventional Heating Technologies. J. Org. Chem., 2008, 73, 7532. https://doi.org/10.1021/jo8013897
D. C. Hancock, N. J. O’ Reilly, G. I. Evan. Synthesis of Peptides for Use as Immunogens. In: Pound JD, ed. Immunochem. Protoc., Totowa, NJ: Humana Press, 1998, 80. https://doi.org/10.1007/978-1-59259-257-9_7
D. C. Hancock, N. J. O’ Reilly, G. I. Evan. Synthesis of Peptides for Use as Immunogens. In: Pound JD, ed. In: Pound JD, ed. Immunochem. Protoc., Totowa, NJ: Humana Press, 2005, 295, 13. https://doi.org/10.1385/1-59259-873-0:013
M. TopuzoĞullari, T. Acar, P. P. Arayici, B. uçar, E. uğurel, E. ş. Abamor, T. arasoğlu, D. balik, S. derman. An insight into the epitope-based peptide vaccine design strategy and studies against COVID-19. Turk. J. Biol., 2020, 44, 215. https://doi.org/10.3906/biy-2006-1
S. Halder, N. Jaiswal, S. C. Balajee, N. Mahata. Efficient production of a novel recombinant fusion protein of EIEC effector IpaD and EGFP: Biophysical characterization and functional studies. Biochim. Biophys. Acta Proteins Proteom., 2025, 1873, 141066. https://doi.org/10.1016/j.bbapap.2025.141066
S Halder, N Jaiswal, H Koley, N Mahata. Cloning, improved expression and purification of invasion plasmid antigen D (IpaD): an effector protein of enteroinvasive Escherichia coli (EIEC). Biotechnol. Genet. Eng. Rev., 2024, 40, 409. https://10.1080/02648725.2023.2184027
M. Gomez-Perosanz, A. Ras-Carmona, E. M. Lafuente, P. A. Reche. Identification of CD8(+) T cell epitopes through proteasome cleavage site predictions. BMC Bioinformatics, 2020, 21. https://doi.org/10.1186/s12859-020-03782-1
S. S. Ella Mae Joy, C. B. Edward, L. E. Fajardo, N. M. O. Odchimar, A. M. Simbulan, F. L. Orosco. Current strategies, advances, and challenges in multi-epitope subunit vaccine development for African swine fever virus. Vet. Integr. Sci., 2024, 23, 1. https://doi.org/10.12982/VIS.2025.011
P. Saxová, S. Buus, S. Brunak, C. Keşmir. Predicting proteasomal cleavage sites: a comparison of available methods. Int. Immunol., 2003, 15, 781. https://doi.org/10.1093/intimm/dxg084
O. W. Liew, S. S. M. Ling, S. Lilyanna, J. P. C. Chong, J. Y. Xia Ng, A. M. Richards. One-Shot Generation of Epitope-Directed Monoclonal Antibodies to Multiple Nonoverlapping Targets: Peptide Selection, Antigen Preparation, and Epitope Mapping. Methods Mol. Biol., 2023, 2578, 121. https://doi.org/10.1007/978-1-0716-2732-7_9
H. X. Lim, J. Lim, S. D. Jazayeri, S. Poppema, C. l. Poh. Development of multi-epitope peptide-based vaccines against SARS-CoV-2. Biomed. J., 2021, 44, 18. https://doi.org/10.1016/j.bj.2020.09.005
P. Kalita, T. Tripathi. Methodological advances in the design of peptide-based vaccines. Drug. Discov. Today, 2022, 27, 1367. https://doi.org/10.1016/j.drudis.2022.03.004
H. Ardestani, S. Nazarian, A. Hajizadeh, D. Sadeghi, E. Kordbacheh. In silico and in vivo approaches to recombinant multi-epitope immunogen of GroEL provides efficient cross protection against S. Typhimurium, S. flexneri, and S. dysenteriae. Mol. Imm., 2022, 144, 96. https://doi.org/10.1016/j.molimm.2022.02.013
M. Shah, S. Rafiq, H. G. Woo. Challenges and considerations in multi-epitope vaccine design surrounding toll-like receptors. Trends Pharmacol. Sci., 2024, 45, 1104. https://doi.org/10.1016/j.tips.2024.10.013
S. Chowdhury, P. Sadhukhan, N. Mahata. Immunoinformatics investigation on pathogenic Escherichia coli proteome to develop an epitope-based peptide vaccine candidate. Mol. Divers., 2024. https://doi.org/10.1007/s11030-024-11034-0
H. Zelba, A. McQueeney, A. Rabsteyn, O. Bartsch, C. Kyzirakos, S. Kayser, J. Harter, P. Latzer, D. Hadaschik, F. Battk, A. D. Hartkopf, S. Biskup.Adjuvant Treatment for Breast Cancer Patients Using Individualized Neoantigen Peptide Vaccination-A Retrospective Observation. Vaccines, 2022, 10. https://doi.org/10.3390/vaccines10111882
P. Patra, M. Bhattacharya, A. R. Sharma, P. Ghosh, G. Sharma, B. C. Patra, B. Mallick, S. S. Lee, C. Chakraborty. Identification and Design of a Next-Generation Multi Epitopes Bases Peptide Vaccine Candidate Against Prostate Cancer: An In Silico Approach. Cell Biochem. Biophys., 2020, 78, 495. https://doi.org/10.1007/s12013-020-00912-7
H. Namdari, F. Rezaei, F. Heidarnejad, M. Y. Maleki, M. Karamigolbaghi. Immunoinformatics Approach to Design a Chimeric CD70-Peptide Vaccine against Renal Cell Carcinoma. J. Immunol. Res., 2024. https://doi.org/10.1155/2024/2875635
M. Saxena, T. U. Marron, J. Kodysh, Jr. J. P. Finnigan, S. Onkar, A. Kaminska, K. Tuballes, R. Guo, R. L. Sabado, M. Meseck, T. J. O’Donnell, R. P. Sebra, S. Parekh, M. D. Galsky, A. Blasquez, G. Gimenez, M. Bicak, C. C. Bozkus, D. D. Zagelbaum, D. Rodriguez, A. A. Villaorduna, K. J. Misiukiewicz, M. R. Posner, B. A. Miles, H. Y. Irie, A. Tiersten, D. B. Doroshow, A. Wolf, J. Mandeli, R. Brody, A. M. Salazar, S. Gnjatic, J. Hammerbacher, E. Schadt, P. Fiedlander, A. Rubinsteyn, N. Bhardwaj. PGV001, a Multi-Peptide Personalized Neoantigen Vaccine Platform: Phase I Study in Patients with Solid and Hematologic Malignancies in the Adjuvant Setting. Cancer Discov., 2025, 15, 930. https://doi.org/10.1158/2159-8290.CD-24-0934
R. J. Malonis, J. R. Lai, O. Vergnolle. Peptide-Based Vaccines: Current Progress and Future Challenges. Chem. Rev., 2020, 120, 3210. https://doi.org/10.1021/acs.chemrev.9b00472
T. J. Wells, T. Esposito, I. R. Henderson, L. I. Labzin. Mechanisms of antibody-dependent enhancement of infectious disease. Nat. Rev. Immunol., 2025, 25,6. https://doi.org/10.1038/s41577-024-01067-9
M. Pang, X. Z. Sun, T. He, H. Yang, J Chen. Clinical Manifestation of Arboviruses in Paediatrics. Rev. Med. Virol. 2025, 35, e70016. https://doi.org/10.1002/rmv.70016
A. Teo, H. D. Tan, T. Loy, P. Y. Chia, C. L. L. Chua. Understanding antibody-dependent enhancement in dengue: Are afucosylated IgG1s a concern? PLoS pathog., 2023, 19. https://doi.org/10.1371/journal.ppat.1011223
S. J. Thomas. Is new dengue vaccine efficacy data a relief or cause for concern? NPJ Vaccines, 2023, 8, 55. https://doi.org/10.1038/s41541-023-00658-2
T. Šuštić, J. V. Coillie, M. D. Larsen, N. I. L. Derksen, Z. Szittner, J. Nouta, W. Wang, T. Damelang, I. Rebergen, F. Linty, R. Visser, J. Y. Mok, D. M. Geerdes, W. J. E. V. Esch, S. W. de Taeye, M. J. V. Gils, L. V. de Watering, C. E. V. der Schoot, M. Wuhrer, T. Rispens. G. Vidarsson. Immunoassay for quantification of antigen-specific IgG fucosylation. EBioMedicine, 2022, 81, 104109. https://doi.org/10.1016/j.ebiom.2022.104109
R. I. Kitney, J. Bell, J. Philp. Build a Sustainable Vaccines Industry with Synthetic Biology. Trends Biotechnol., 2021, 39, 866. https://doi.org/10.1016/j.tibtech.2020.12.006
O. M. Oyedele. The Cobra Effect: Exploring the Dark Side of Innovation and Environmental Sustainability in Developing Nations. In: Singh R, Joshi A, Filho WL, Khan S, eds. Zero Carbon Industry, Eco-Innovation and Environmental Sustainability. Cham: Springer Nature Switzerland., 2025, 251.
https://doi.org/10.1007/978-3-031-80220-1_15
S. Yadav, D. S. Vora, D. Sundar, J. K. Dhanjal. TCR-ESM: Employing protein language embeddings to predict TCR-peptide-MHC binding. Comput. Struct. Biotechnol. J., 2024, 23, 165. https://doi.org/10.1016/j.csbj.2023.11.037
Y. Zhao, J. J. Yu, Y. Su, E. Ma, J. Wang, S. Jiang, C. Wei, D. Li, Z. Huang, G. Cheng, H. Ren, J. Feng. A unified deep framework for peptide–major histocompatibility complex–T cell receptor binding prediction. Nat. Mach. Intell., 2025. https://doi.org/10.1038/s42256-025-01002-0
Y. Wei, T. Qiu, Y. Ai, Y. Zhang, J. Xie, D. Zhang, X. Luo, X. Sun, X. Wang, J. Qiu. Advances of computational methods enhance the development of multi-epitope vaccines. Brief. Bioinform., 2025, 26. https://doi.org/10.1093/bib/bbaf055
M. Rahman. Editorial: design considerations for future personalized vaccination approaches. Nanomedicine, 2025, 20, 329. https://doi.org/10.1080/17435889.2024.2419816
A. Vasou, N. Sultanoglu, S. Goodbourn, R. E. Randall, L. G. Kostrikis. Targeting Pattern Recognition Receptors (PRR) for Vaccine Adjuvantation: From Synthetic PRR Agonists to the Potential of Defective Interfering Particles of Viruses. Viruses, 2017, 9. https://doi.org/10.3390/v9070186
H. Afzal, A. Murtaza, L. T. Cheng. Structural engineering of flagellin as vaccine adjuvant: quest for the minimal domain of flagellin for TLR5 activation. Mol. Biol. Rep., 2025, 52. https://doi.org/10.1007/s11033-024-10146-y
T. Lu, S. Das, D. R. Howlader, A. Jain, G. Hu, Z. K. Dietz, Q. Zheng, S. S. K. Ratnakaram, S. K. Whittier, D. J. Varisco, R. K. Ernst, W. D. Picking, W. L. Picking. Impact of the TLR4 agonist BECC438 on a novel vaccine formulation against Shigella spp. Front. Immunol., 2023, 14. https://doi.org/10.3389/fimmu.2023.1194912
C. G. Park. Vaccine strategies utilizing C-type lectin receptors on dendritic cells in vivo. Clin. Exp. Vaccine Res., 2014, 3, 149. https://doi.org/10.7774/cevr.2014.3.2.149
H. Y. Yong, D. Luo. RIG-I-Like Receptors as Novel Targets for Pan-Antivirals and Vaccine Adjuvants Against Emerging and Re-Emerging Viral Infections. Front. Immunol., 2018, 9. https://doi.org/10.3389/fimmu.2018.01379
A. Atalis, M. C. Keenum, B. Pandey, A. Beach, P. Pradhan, C. Vantucci, L. O'Farrell, R. Noel, R. Jain, J. Hosten, C. Smith, L. Kramer, A. Jimenez, M. A. Ochoa, D. Frey, K. Roy. Nanoparticle-delivered TLR4 and RIG-I agonists enhance immune response to SARS-CoV-2 subunit vaccine. J. Control Release., 2022, 347, 476. https://doi.org/10.1016/j.jconrel.2022.05.023
Q. Li, Z. Li, N. Deng, F. Ding, Y. Li, H. Cai. Built-in adjuvants for use in vaccines. Eur. J. Med. Chem., 2022, 227. https://doi.org/10.1016/j.ejmech.2021.113917
P. Bhardwaj, G. P. Biswas, N. Mahata, S. Ghanta, B. Bhunia. Exploration of binding mechanism of triclosan towards cancer markers using molecular docking and molecular dynamics. Chemosphere, 2022, 293, 133550. https://doi.org/10.1016/j.chemosphere.2022.133550
R. López, N. Díaz, E. Francisco, A. Martín-Pendás, D. Suárez. QM/MM Energy Decomposition Using the Interacting Quantum Atoms Approach. J. Chem. Inf. Model., 2022, 62, 1510. https://doi.org/10.1021/acs.jcim.1c01372
J. R. Perilla, B. C. Goh, C. K. Cassidy, B. Liu, R. C. Bernardi, T. Rudack, H. Yu, Z. Wu, K. Schulten. Molecular dynamics simulations of large macromolecular complexes. Curr. Opin. Struct. Biol., 2015, 31, 64. https://doi.org/10.1016/j.sbi.2015.03.007
G. A. Poland, J. de la Fuente. Quantum vaccinology: A new science and epistemological abstraction framework for developing new vaccines and understanding the generation of the immune response. Vaccine, 2025, 46. https://doi.org/10.1016/j.vaccine.2024.126641
J. M. Chow. Quantum computing requires high-performance software. Science, 2025, 387. https://doi.org/10.1126/science.adt0019
I. Kolossváry. A Fresh Look at the Normal Mode Analysis of Proteins: Introducing Allosteric Co-Vibrational Modes. JACS Au., 2024, 4, 1303. https://doi.org/10.1021/jacsau.4c00109
A. G. Moulick, R. Patel, A. Onyema, S. M. Loverde. Unveiling nucleosome dynamics: A comparative study using all-atom and coarse-grained simulations enhanced by principal component analysis. J. Chem. Phys., 2025, 162. https://doi.org/10.1063/5.0246977
M. Wang, A. Ma, H. Wang, X. Lou. Atomic molecular dynamics simulation advances of de novo-designed proteins. Q. Rev. Biophys., 2024, 57. https://doi.org/10.1017/S0033583524000131
J. Rizo, L. Sari, Y. Qi, W. Im, M. M. Lin. All-atom molecular dynamics simulations of Synaptotagmin-SNARE-complexin complexes bridging a vesicle and a flat lipid bilayer. eLife, 2022, 11. https://doi.org/10.7554/eLife.76356
S. Phogat, J. Yadav, D. Chaudhary, R. Jaiwal, P. K. Jaiwal. Synthesis of an Adjuvant-Free Single Polypeptide-Based Tuberculosis Subunit Vaccine that Elicits In Vivo Immunogenicity in Rats. Mol. Biotechnol., 2025, (online). https://doi.org/10.1007/s12033-025-01431-7
W. Z. Huang, W. H. Hu, Y. Wang, J. Chen. Z. Hu, J. Zhou, L. Liu, W. Qiu, F. Tang, S. Zhang, Y. Ouyang, Y. Ye, G. Xu, J. Long, Z. Zeng. A Mathematical Modelling of Initiation of Dendritic Cells-Induced T Cell Immune Response. Int. J. Biol. Sci., 2019, 15, 1396. https://doi.org/10.7150/ijbs.33412
G. Gasperini, N. Baylor, S. Black, J. Cramer, G. de Lannoy, P. Denoel. M. Feinberg, T. Helleputte, G. Kang, W. R. Schief, L, Stuart, C. Weller, M. Zwierzyna, R. Rappuoli. Vaccinology in the artificial intelligence era. Sci. Transl. Med., 2025, 17. https://doi.org/10.1126/scitranslmed.adu3791
L. Wu, H. Lin, Y. Huang, Z. Gao, C. tan, Y. Liu, T. Wu, S. Z. Li. Relation-aware equivariant graph networks for epitope-unknown antibody design and specificity optimization. Paper presented at: Proceedings of the AAAI Conference on Artificial Intelligence, 2025. https://doi.org/10.48550/arXiv.2501.00013
G. Giusmin. The immune system. In: The Anatomy and Physiology Textbook for Midwives. Routledge, 2025, 197. ISBN-9781032130842.
I. Rastogi, D. Jeon, J. E. Moseman, A. Muralidhar. H. K. Potluri, D. G. McNeel. Role of B cells as antigen presenting cells. Front. Immunol., 2022, 13. https://doi.org/10.3389/fimmu.2022.954936
R. J. Ji, M. Y. Wang, Y. Zhang. Precision epitope editing: A path to advanced immunotherapies. Cell Insight, 2025, 4. https://doi.org/10.1016/j.cellin.2024.100226
C. L. P. Eng, T. W. Tan, J. C. Tong. Immunoinformatics Databases. In: Ranganathan S, Gribskov M, Nakai K, Schönbach C, eds. Encyclopedia of Bioinformatics and Computational Biology. Oxford: Academic Press, 2019, 931. https://doi.org/10.1016/B978-0-12-809633-8.20469-X
J. Sidney, B. Peters, A. Sette. Epitope prediction and identification- adaptive T cell responses in humans. Semin. Immunol., 2020, 50. https://doi.org/10.1016/j.smim.2020.101418
J. M. Gershoni, A. Roitburd-Berman, D. D. Siman-Tov, N. T. Freund, Y. Weiss. Epitope mapping: the first step in developing epitope-based vaccines. BioDrugs, 2007, 21, 145. https://doi.org/10.2165/00063030-200721030-00002
R. Viswanathan, M. Carroll, A. Roffe, J. E. Fajardo, A. Fiser. Computational prediction of multiple antigen epitopes. Bioinformatics, 2024, 40. https://doi.org/10.1093/bioinformatics/btae556
N. Lalinde-Ruiz, L. C. Martínez-Enriquez, D. A. Gutierrez, H. H. Nieto, L. F. Niño, C. A. Parra-López. Methodological approach to identify immunogenic epitopes candidates for vaccines against emerging pathogens tailored to defined HLA populations. Comput. Biol. Chem., 2025, 116. https://doi.org/10.1016/j.compbiolchem.2025.108389
H. W. Davidson, C. Watts. Epitope-directed processing of specific antigen by B lymphocytes. J. Cell Biol., 1989, 109, 85. https://doi.org/10.1083/jcb.109.1.85
R. Vita, N. Blazeska, D. Marrama, IEDB Curation Team Members, S. Duesing, J. Bennett, J. Greenbaum, M. D. A. Mendes, J. Mahita, D. K. Wheeler, J. R. Cantrell, J. A. Overton, D. A. Natale, A. Sette, B. Peters. The Immune Epitope Database (IEDB): 2024 update. Nucleic Acids Res., 2024, 53, D436. https://doi.org/10.1093/nar/gkae1092
B. Liu, M. Bai, F. Zheng, M. Yan, E. Huang, J. Wen, Y. Li, J. Wang. The Identification of Dual T-Cell and B-Cell Epitopes Within Viral Proteins Utilizing a Comprehensive Peptide Array Approach. Vaccines, 2025, 13, 239. https://doi.org/10.3390/vaccines13030239
M. Bhattacharjee, M. Banerjee, A. Mukherjee. Advanced in silico design of an optimized multi-epitope peptide vaccine employing immunoinformatics and reverse vaccinology strategies on the model of Listeria monocytogenes. J. Proteins Proteom., 2025, 16, 213. https://doi.org/10.1007/s42485-025-00185-9
A. Saihar, A. R. Yaseen, M. Suleman, R. Parveen, H. Bashir. From bytes to bites: In-silico creation of a novel multi-epitope vaccine against Murray Valley Encephalitis Virus. Microb. Pathog., 2025, 198. https://doi.org/10.1016/j.micpath.2024.107171
Z. Bayat, Z. Majidi, A. Taherkhani, L. Samie. Engineering a Novel T-Cell Epitope-Based Vaccine Targeting PRAME in Cancer Immunotherapy. Pept. Sci., 2025, 117. https://doi.org/10.1002/pep2.24393
V. Vaghasia, L. K. Snehkant, P. Saumya, J. Das. Epitopes mapping for identification of potential cross-reactive peptide against leptospirosis. J. Biomol. Struct. Dyn., 2025, 43, 20. https://doi.org/10.1080/07391102.2023.2279285
V. Ahlawat, K. Sura, M. Dangi, A. K. Chhillar. Designing of multi-valent epitope vaccine displaying interactions with diverse HLA alleles against Candida auris using immuno-informatics. J. Genet. Eng. Biotechnol., 2025, 23. https://doi.org/10.1016/j.jgeb.2025.100474
D. R. L. Agnila, R. Jain, M. J. Diaz, T. R. Hudlock, R. A. Eakins, A. Chobrutskiy, B. I. Chobrutskiy, G. Blanck. TCR CDR3 chemical complementarity to HPV epitopes is associated with a better outcome for cervical cancer. Mamm. Genome., 2025, 36, 683. https://doi.org/10.1007/s00335-025-10127-x
M. Nahian, M. Shahab, M. R. Khan, S. Akash, T. A. Banu, M. H. Sarkar, B. Goswami, S. F. Chowdhury, M. A. Islam, A. A. Rus’d, S. Bgum, A. Habib, A. A. Shaikh, J. I. N. Oliveira, S. Akter. Development of a broad-spectrum epitope-based vaccine against Streptococcus pneumoniae. PloS one, 2025, 20. https://doi.org/10.1371/journal.pone.0317216
F. F. Gonzalez-Galarza, A. McCabe, E. J. M. Dos Santos,J. Jones, L. Takeshita, N. D. Ortega-Rivera, G. M. D. Cid-Pavon, K. Ramsbottom, G. Ghattoraya, A. Alfirevic, D. Middleton, A. R. Jones. Allele frequency net database (AFND) 2020 update: gold-standard data classification, open access genotype data and new query tools. Nucleic Acids Res., 2019, 48, D783. https://10.1093/nar/gkz1029
I. Doytchinova, D. Flower. VaxiJen: a server for prediction of protective antigens, tumour antigens and subunit vaccines. BMC Bioinformatics, 2007, 8, 4. https://doi.org/10.1186/1471-2105-8-4
T. L. Nguyen, T. B. Nguyen, H. Kim. Computational identification of B and T-cell epitopes for designing a multi-epitope vaccine against SARS-CoV-2 spike glycoprotein. J. Struct. Biol., 2025, 217, 108. https://doi.org/10.1016/j.jsb.2025.108177
R. D. Huby, R. J. Dearman, I. Kimber. Why are some proteins allergens? Toxicol. Sci., 2000, 55, 235. https://doi.org/10.1093/toxsci/55.2.235
F. Huan, S. Gao, Y. Gu, L. Ni, M. Wu, Y. Li, M. Liu, Y. Yang, A. Xiao, G. Liu. Molecular Allergology: Epitope Discovery and Its Application for Allergen-Specific Immunotherapy of Food Allergy. Clinic. Rev. Allerg. Immunol., 2025, 68, 37. https://doi.org/10.1007/s12016-025-09052-3
I. Dimitrov, I. Bangov, D. R. Flower, I. Doytchinova. AllerTOP v.2--a server for in silico prediction of allergens. J. Mol. Model., 2014, 20, 2278. https://doi.org/10.1007/s00894-014-2278-5
M. N. Nguyen, N. L. Krutz, V. Limviphuvadh, A. L. Lopata, G. F. Gerberick, S. Maurer-Stroh. AllerCatPro 2.0: a web server for predicting protein allergenicity potential. Nucleic Acids Res., 2022, 50, W36. https://doi.org/10.1093/nar/gkac446
S. Saha, G. P. Raghava. AlgPred: prediction of allergenic proteins and mapping of IgE epitopes. Nucleic Acids Res., 2006, 34, W202. https://doi.org/10.1093/nar/gkl343
I. Dimitrov, L. Naneva, I. Doytchinova, I. Bangov. AllergenFP: allergenicity prediction by descriptor fingerprints. Bioinformatics, 2014, 30, 846. https://doi.org/10.1093/bioinformatics/btt619
I. Dimitrov, I. Doytchinova. An Alignment-Independent Platform for Allergenicity Prediction. In: Tomar N, ed. Immunoinformatics, New York, NY: Springer US. 2020, 147. https://doi.org/10.1007/978-1-0716-0389-5_5
T. Sisay, N. Maina, S. Wachira, V. A. Mobegi. In-silico evaluation of fungal and bacterial L-asparaginases allergenicity. Inform. Med. Unlocked, 2023, 43, 101398. https://doi.org/10.1016/j.imu.2023.101398
S. Basith, N. T. Pham, B. Manavalan, G. Lee. SEP-AlgPro: An efficient allergen prediction tool utilizing traditional machine learning and deep learning techniques with protein language model features. Int. J. Biol. Macromol., 2024, 273, 133085. https://doi.org/10.1016/j.ijbiomac.2024.133085
N. Cristianini, E. Ricci. Support Vector Machines. In: Kao M-Y, ed. Encyclopedia of Algorithms. Boston, MA: Springer US, 2008, 928. https://doi.org/10.1007/978-0-387-30162-4_415
S. Gupta, P. Kapoor, K. Chaudhary, A. Gautam, R. Kumar, OSDDC, G. P. S. Raghava. In silico approach for predicting toxicity of peptides and proteins. PloS one, 2013, 8. https://doi.org/10.1371/journal.pone.0073957
G. Reena, R. Ranjani, K. D. Goutham, K. Sangeetha. In silico screening of plant peptides against the envelope protein of dengue virus. Trop. Biomed., 2023, 40, 124. https://doi.org/10.47665/tb.40.2.001
X. Pan, J. Zuallaert, X. Wang, H. Shen, E. P. Campos, D. O. Marushchak, W. D. Neve. ToxDL: deep learning using primary structure and domain embeddings for assessing protein toxicity. Bioinformatics, 2021, 36, 5159. https://doi.org/10.1093/bioinformatics/btaa656
J. N. Clifford, M. H. Høie, S. Deleuran, B. Peters, M. Nielsen, P. Marcatili. BepiPred-3.0: Improved B-cell epitope prediction using protein language models. Protein Sci., 2022, 31, e4497. https://doi.org/10.1002/pro.4497
S. Saha, G. P. Raghava. Prediction of continuous B-cell epitopes in an antigen using recurrent neural network. Proteins, 2006, 65, 40. https://doi.org/10.1002/prot.21078
H. Singh, H. R. Ansari, G. P. Raghava. Improved method for linear B-cell epitope prediction using antigen's primary sequence. PloS one, 2013, 8, e62216. https://doi.org/10.1371/journal.pone.0062216
M. Collatz, F. Mock, E. Barth, M. Hölzer, K. Sachse, M Marz. EpiDope: a deep neural network for linear B-cell epitope prediction. Bioinformatics, 2020, 37, 448. https://doi.org/10.1093/bioinformatics/btaa773
Y. El-Manzalawy, D. Dobbs, V. Honavar. Predicting linear B-cell epitopes using string kernels. J. Mol. Recognit., 2008, 21, 243. https://doi.org/10.1002/jmr.893
M. H. Høie, F. S. Gade, J. M. Johansen, C. Würtzen, O. Winther, M. Nielsen, P. Marcatili. DiscoTope-3.0: improved B-cell epitope prediction using inverse folding latent representations. Front. Immunol., 2024, 15, 1322712. https://doi.org/10.3389/fimmu.2024.1322712
J. Ponomarenko, H. H. Bui, W. Li, N. Fusseder, P. E. Bourne, A. Sette, B. Peters. ElliPro: a new structure-based tool for the prediction of antibody epitopes. BMC bioinformatics, 2008, 9, 514. https://doi.org/10.1186/1471-2105-9-514
M. J. Sweredoski, P. Baldi. PEPITO: improved discontinuous B-cell epitope prediction using multiple distance thresholds and half sphere exposure. Bioinformatics, 2008, 24, 1459. https://doi.org/10.1093/bioinformatics/btn199
U. Kulkarni-Kale, S. Bhosle, A. S. Kolaskar. CEP: a conformational epitope prediction server. Nucleic Acids Res., 2005, 33, W168. https://doi.org/10.1093/nar/gki460
J. Sun, D. Wu, T. Xu, X. Wang, X. Xu, L. Tao, Y. X. Li, Z. W. Cao. SEPPA: a computational server for spatial epitope prediction of protein antigens. Nucleic Acids Res., 2009, 37, W612. https://doi.org/10.1093/nar/gkp417
M. Atanasova, A. Patronov, I. Dimitrov, D. R. Flower, I. Doytchinova. EpiDOCK: a molecular docking-based tool for MHC class II binding prediction. Protein Eng. Des. Sel., 2013, 26, 631. https://doi.org/10.1093/protein/gzt018
P. A. Reche, E. L. Reinherz. PEPVAC: a web server for multi-epitope vaccine development based on the prediction of supertypic MHC ligands. Nucleic Acids Res., 2005, 33, W138. https://doi.org/10.1093/nar/gki357
I. Dimitrov, P. Garnev, D. R. Flower, I. Doytchinova. EpiTOP—a proteochemometric tool for MHC class II binding prediction. Bioinformatics, 2010, 26, 2066. https://doi.org/10.1093/bioinformatics/btq324
P. Guan, C. K. Hattotuwagama, I. A. Doytchinova, D. R. Flower. MHCPred 2.0: an updated quantitative T-cell epitope prediction server. Appl. Bioinformatics, 2006, 5, 55. https://doi.org/10.2165/00822942-200605010-00008
C. A. Mirkin, R. Langer, M. Mrksich, A. Margolin, A., S. H. Petrosko, & N. Artzi. Blueprints for Better Drugs: The Structural Revolution in Nanomedicine. ACS Nano, 2025, 19, 18889. https://doi:10.1021/acsnano.5c06380
D. T. Ouologuem, F. O. Maiga, A. Dara, A. Djimdé, D. A. K. Traore, E. Nji. Hands-on training in structural biology, a tool for sustainable development in Africa series 4. Biology open. 2022, 11. http://doi.org/10.1242/bio.059487
E. Nji, A. F. A. Moumbock, K. C. Cramer, N. V. Rüffin, J. Davis, O. A. Asojo, J. J. Griese, A. A. Larbi, M. N. Fodje. Supporting structural biologists in Africa requires resources and capacity building. Nat. Struct. Mol. Biol., 2024, 31, 1814. https://doi.org/10.1038/s41594-024-01438-9
J. Jumper, R. Evans, A. Pritzel, T. Green, M. Figurnov, O. Ronneberger, K. Tunyasuvunakool, R. Bates., A. Žídek, A. Potapenko, A. Bridgland, C. Meyer, S. A. A. Kohl, A. J. Ballard, A. Cowie, B. Romera-Paredes, S. Nikolov, R. Jain, J. Adler, T. Back, S. Petersen, D. Reiman, E. Clancy, M. Zielinski, M. Sterinegger, M. Pacholska, T. Berghammer, S. Bodenstein, D. Silver, O. Vinyals, A. W. Senior, K. Kavukcuoglu, P. Kohli, D. Hassabis. Highly accurate protein structure prediction with AlphaFold. Nature, 2021, 596, 583. https://doi.org/10.1038/s41586-021-03819-2
M. Mirdita, K. Schütze, Y. Moriwaki, L. Heo, S. Ovchinnikov, M. Steinegger. ColabFold: making protein folding accessible to all. Nat. Methods, 2022, 19, 679. https://doi.org/10.1038/s41592-022-01488-1
S. Park, S. Myung, M. Baek. Advancing protein structure prediction beyond AlphaFold2. Curr. Opin. Struct. Biol., 2025, 90, 102985. https://doi.org/10.1016/j.sbi.2025.102985
M. Torrisi, G. Pollastri, Q. Le. Deep learning methods in protein structure prediction. Comput. Struct. Biotechnol. J., 2020, 18, 1301. https://doi.org/10.1016/j.csbj.2019.12.011
J. Jänes, P. Beltrao. Deep learning for protein structure prediction and design—progress and applications. Mol. Syst. Biol., 2024, 20, 162. https://doi.org/10.1038/s44320-024-00016-x
C. Chen, X. Chen, A. Morehead, T. Wu, J. Cheng. 3D-equivariant graph neural networks for protein model quality assessment. Bioinformatics, 2023, 39. https://doi.org/10.1093/bioinformatics/btad030
L. Kong, F. Ju, W. M. Zheng, J. Zhu, S. Sun, J. Xu, D. Bu. ProALIGN: Directly Learning Alignments for Protein Structure Prediction via Exploiting Context-Specific Alignment Motifs. J. Comput. Biol., 2022, 29, 92. https://doi.org/10.1089/cmb.2021.0430
C. Zhang, Q. Wang, Y. Li, A. Teng, G. Hu, Q. Wuyun, W. Zheng. The Historical Evolution and Significance of Multiple Sequence Alignment in Molecular Structure and Function Prediction. Biomolecules, 2024, 14, 1531. https://doi.org/10.3390/biom14121531
R. S. Aal, E. Ali, J. Meng, M. E. I. Khan, X. Jiang. Machine learning advancements in organic synthesis: A focused exploration of artificial intelligence applications in chemistry. Artif. Intell. Chem., 2024, 2, 100049. https://doi.org/10.1016/j.aichem.2024.100049
J. Koehler Leman, G. Künze. Recent Advances in NMR Protein Structure Prediction with ROSETTA. Int. J. Mol. Sci., 2023, 24, 7835. https://doi.org/10.3390/ijms24097835
M. Baek, F. DiMaio, I. Anishchenko, J. Dauparas, S. Ovchinnikov, G. R. Lee, J. Wang, Q. Cong, L. N. Kinch, R. D. Schaeffer, G. R. Lee, J. Wang, Q. Cong, L. N. Kinch, R. D. Schaeffer, C. Millán, H. Park, C. Adams, C. R. Glassman, A. DeGiovanni, J. H. Pereira, A. V. Rodrigues, A. A. V. Dijk, A. C. Ebrecht, D. J. Opperman, T. Sagmeister, C. Buhlheller, T. Pavkov-Keller, M. K. Rathinaswamy, U. Dalwadi, C. K. Yip, J. E. Burke, K. C. Garcia, N. V. Grishin, P. D. Adams, R. J. Read , D. Bekar. Accurate prediction of protein structures and interactions using a three-track neural network. Science, 2021, 373, 871. https://doi.org/10.1126/science.abj8754
Q. Wuyun, Y Chen, Y Shen, Y. Cao, G. Hu, W. Cui, J. Gao, W. Zheng. Recent Progress of Protein Tertiary Structure Prediction. Molecules, 2024, 29, 832. https://doi.org/10.3390/molecules29040832
J Rey, S Murail, S de Vries, P Derreumaux, P Tuffery. PEP-FOLD4: a pH-dependent force field for peptide structure prediction in aqueous solution. Nucleic Acids Res., 2023, 51, W432. https://doi.org/10.1093/nar/gkad376
C. T. Mant, R. S. Hodges. Design of peptide standards with the same composition and minimal sequence variation to monitor performance/selectivity of reversed-phase matrices. J. Chromatogr. A., 2012, 1230, 30. https://doi.org/10.1016/j.chroma.2012.01.053
A. Mondal, L. Chang, A. Perez. Modelling peptide-protein complexes: docking, simulations and machine learning. QRB Discov., 2022, 3, e17. https://doi.org/10.1017/qrd.2022.14
B. Raveh, N. London, O. Schueler-Furman. Sub-angstrom modeling of complexes between flexible peptides and globular proteins. Proteins, 2010, 78, 2029. https://doi.org/10.1002/prot.22716
M. Ciemny, M. Kurcinski, K. Kamel, A. Kolinski, N. Alam, O. Schueler-Furman, S. Kmiecik. Protein–peptide docking: opportunities and challenges. Drug Discov. Today, 2018, 23, 1530. https://doi.org/10.1016/j.drudis.2018.05.006
O. Trott, A. J. Olson. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem., 2010, 31, 455. https://doi.org/10.1002/jcc.21334
M. L. Verdonk, J. C. Cole, M. J. Hartshorn, C. W. Murray, R. D. Taylor. Improved protein-ligand docking using GOLD. Proteins, 2003, 52, 609. https://doi.org/10.1002/prot.10465
J. Patra, A. K. Keshari, R. R. Bhandare, A. B. Shaik, M. Parrot, S. Lin. Discovery of Novel Multiangiogenic Agents Targeting VEGFR2, EphB4, FGFR-1, and TIE-2: Receptor-Based Pharmacophore Modeling, Virtual Screening, and Molecular Modeling Studies. ACS omega, 2025, 10, 13880. https://doi.org/10.1021/acsomega.4c08366
S. Huang, D. Gu, W. Xiong. Exploring the functional and prognostic roles of EPHX4 in pancreatic cancer: Insights from bioinformatics and experimental validation. Gene, 2025, 959, 149504. https://doi.org/10.1016/j.gene.2025.149504
H. Lee, L. Heo, M. S. Lee, C. Seok. GalaxyPepDock: a protein-peptide docking tool based on interaction similarity and energy optimization. Nucleic Acids Res., 2015, 43, W431. https://doi.org/10.1093/nar/gkv495
A. Obarska-Kosinska, A. Iacoangeli, R. Lepore, A. Tramontano. PepComposer: computational design of peptides binding to a given protein surface. Nucleic Acids Res., 2016, 44, W522. https://doi.org/10.1093/nar/gkw366
I. Johansson-Åkhe, C. Mirabello, B. Wallner. InterPep2: global peptide–protein docking using interaction surface templates. Bioinformatics, 2020, 36, 2458. https://doi.org/10.1093/bioinformatics/btaa005
R. V. Honorato, M. E. Trellet, B. Jiménez-García, Jörg J. Schaarschmidt, M. Giulini, V. Reys, P. I. Koukos, J. P. G. L. M. Rodrigues, E. Karaca, G. C. P. V. Zundert, J. Roel-Touris, C. W. V. Noort, Z. Jandová, A. S. J. Melquiond, A. M. J. J. Bonvin. The HADDOCK2.4 web server for integrative modeling of biomolecular complexes. Nat. Protoc., 2024, 19, 3219. https://doi.org/10.1038/s41596-024-01011-0
P. Zhou, B. Jin, H. Li, S. Y. Huang. HPEPDOCK: a web server for blind peptide-protein docking based on a hierarchical algorithm. Nucleic Acids Res., 2018, 46, W443. https://doi.org/10.1093/nar/gky357
D. A. Antunes, M. Moll, D. Devaurs, K. R. Jackson, G. Lizée, L. E. Kavraki. DINC 2.0: A New Protein-Peptide Docking Webserver Using an Incremental Approach. Cancer Res., 2017, 77, e55. https://doi.org/10.1158/0008-5472.can-17-0511
E. Donsky, H. J. Wolfson. PepCrawler: a fast RRT-based algorithm for high-resolution refinement and binding affinity, estimation of peptide inhibitors. Bioinformatics, 2011, 27, 2836. https://doi.org/10.1093/bioinformatics/btr498
B. Raveh, N. London, L. Zimmerman, O. Schueler-Furman. Rosetta FlexPepDock ab-initio: simultaneous folding, docking and refinement of peptides onto their receptors. PloS one 2011, 6, e18934. https://doi.org/10.1371/journal.pone.0018934
X. Xu, C. Yan, X. Zou. MDockPeP: An ab-initio protein-peptide docking server. J. Comput. Chem., 2018, 39, 2409. https://doi.org/10.1002/jcc.25555
X. Xu, X. Zou. Predicting Protein–Peptide Complex Structures by Accounting for Peptide Flexibility and the Physicochemical Environment. J. Chem. Inf. Model., 2022, 62, 27. https://doi.org/10.1021/acs.jcim.1c00836
C. E. M. Schindler, S. J. de Vries, M. Zacharias. Fully Blind Peptide-Protein Docking with pepATTRACT. Structure, 2015, 23, 1507. https://doi.org/10.1016/j.str.2015.05.021
M. Kurcinski, M. Jamroz, M. Blaszczyk, A. Kolinski, S. Kmiecik. CABS-dock web server for the flexible docking of peptides to proteins without prior knowledge of the binding site. Nucleic Acids Res., 2015, 43, W419. https://doi.org/10.1093/nar/gkv456
N. Alam, O. Goldstein, B. Xia, K. A. Porter, D. Kozakov, O. Schueler-Furman. High-resolution global peptide-protein docking using fragments-based PIPER-FlexPepDock. PLoS. Comput. Biol., 2017, 13, e1005905. https://doi.org/10.1371/journal.pcbi.1005905
Y. Zhang, M. F. Sanner. AutoDock CrankPep: combining folding and docking to predict protein-peptide complexes. Bioinformatics, 2019, 35, 5121. https://doi.org/10.1093/bioinformatics/btz459
A. Khramushin, Z. Ben-Aharon, T. Tsaban, J. K. Varga, O. Avraham, O. Schueler-Furman. Matching protein surface structural patches for high-resolution blind peptide docking. Proc. Natl. Acad. Sci., 2022, 119, e2121153119. https://doi.org/10.1073/pnas.2121153119
E. Ayan. Computational insights into the allosteric behavior of mini proinsulin driven by C peptide mobility. Sci. Rep., 2025, 15, 8065. https://doi.org/10.1038/s41598-025-92799-8
S. Subhadarshini, H. Tandon, N. Srinivasan, R. Sowdhamini. Normal Mode Analysis Elicits Conformational Shifts in Proteins at Both Proximal and Distal Regions to the Phosphosite Stemming from Single-Site Phosphorylation. ACS omega, 2024, 9, 24520. https://doi.org/10.1021/acsomega.4c00523
R. Ma, B. Du, C. Shi, L. Wang, F. Zeng, J. Han, H. Guan, Y. Wang, K. Yan. Molecular basis for the regulation of human phosphorylase kinase by phosphorylation and Ca2+. Nat. Commun., 2025, 16, 3020. https://doi.org/10.1038/s41467-025-58363-8
B. Ganesh, A. Banerjee, L. Guruprasad. Evaluating the ability of in silico identified hit compounds to bind Staphylococcus aureus LcpA(SA) using steered molecular dynamics simulations. Mol. Divers., 2025. https://doi.org/10.1007/s11030-025-11155-0
S. P. D. Sidhanta, R. Sowdhamini, N. Srinivasan. Comparative analysis of permanent and transient domain-domain interactions in multi-domain proteins. Proteins, 2025, 93, 197. https://doi.org/10.1002/prot.26581
E. Ayan, M. Türk, Ö. Tatlı, S. Bostan, E. Telek, B. Dingiloğlu, B. Z. Doğan, M. I. Alp, Ahmet Katı, Gizem Dinler-Doğanay, H. Demirci. X-ray crystallographic and hydrogen deuterium exchange studies confirm alternate kinetic models for homolog insulin monomers. PloS one, 2025, 20, e0319282. https://doi.org/10.1371/journal.pone.0319282
S. Subhadarshini, S. Sahoo, M. K. Jolly, M. Rashid. An integrative molecular systems approach unravels mechanisms underlying biphasic nitrate uptake by plant nitrate transporter NRT1.1. bioRxiv. 2025. https://doi.org/10.1101/2025.01.28.635294
D. M. Krüger, A. Ahmed, H. Gohlke. NMSim web server: integrated approach for normal mode-based geometric simulations of biologically relevant conformational transitions in proteins. Nucleic Acids Res., 2012, 40, W310. https://doi.org/10.1093/nar/gks478
J. R. López-Blanco, J. I. Aliaga, E. S. Quintana-Ortí, P. Chacón. iMODS: internal coordinates normal mode analysis server. Nucleic Acids Res., 2014, 42 (Web Server issue), W271. https://doi.org/10.1093/nar/gku339
E. Eyal, G. Lum, I. Bahar. The anisotropic network model web server at 2015 (ANM 2.0). Bioinformatics, 2015, 31, 1487. https://doi.org/10.1093/bioinformatics/btu847
D. Van Der Spoel, E. Lindahl, B. Hess, G. Groenhof, A. E. Mark, H. J. Berendsen. GROMACS: fast, flexible, and free. J. Comput. Chem., 2005, 26, 1701. https://doi.org/10.1002/jcc.20291
D. A. Case, H. M. Aktulga, K. Belfon, D. S. Cerutti, G. A. Cisneros, V. W. D. Cruzeiro, N. Forouzesh, T. J. Giese, A. W. Götz, H. Gohlke, S. Izadi, K. Kasavajhala, M. C. Kaymak, E. King, T. Kurtzman, T. S. Lee, P. Li, J. Liu, T. Luchko, R. Luo, M. Manathunga, M. R. Machado, H. M. Nguyen, K. A. O'Hearn, A. V. Onufriev, F. Pan, S. Pantano , R. Qi, A. Rahnamoun, A. Risheh, S. Schott-Verdugo, A. Shajan, J. Swails, J. Wang, H. Wei, X. Wu , Y. Wu, S. Zhang, S. Zhao, Q. Zhu, 3rd T. E. Cheatham, D. R. Roe, A. Roitberg, C. Simmerling, D. M. York, M. C. Nagan, Jr. K. M. Merz. AmberTools. J. Chem. Inf. Model., 2023, 63, 6183. https://doi.org/10.1021/acs.jcim.3c01153
B. R. Brooks, 3rd C. L. Brooks, Jr. A. D. Mackerell, L. Nilsson, R. J. Petrella, B. Roux, Y. Won, G. Archontis, C. Bartels, S. Boresch, A. Caflisch, L. Caves, Q. Cui, A. R. Dinner, M. Feig, S. Fischer, J. Gao, M. Hodoscek, W. Im, K. Kuczera, T. Lazaridis, J. Ma, V. Ovchinnikov, E. Paci, R. W. Pastor, C. B. Post, J. Z. Pu, M. Schaefer, B. Tidor, R. M. Venable, H. L. Woodcock, X. Wu, W. Yang, D. M. York, M. Karplus. CHARMM: the biomolecular simulation program. J. Comput. Chem., 2009, 30, 1545. https://doi.org/10.1002/jcc.21287
R. Qi, G. Wei, B. Ma, R. Nussinov. Replica Exchange Molecular Dynamics: A Practical Application Protocol with Solutions to Common Problems and a Peptide Aggregation and Self-Assembly Example. Methods Mol. Biol., 2018, 1777, 101. https://doi.org/10.1007/978-1-4939-7811-3_5
G. Boggarapu, A. Banerjee, L. Guruprasad. Evaluating the ability of in silico identified hit compounds to bind Staphylococcus aureus LcpASA using steered molecular dynamics simulations. Mol. Divers., 2025. https://doi.org/10.1007/s11030-025-11155-0
J. Wang, A. Ishchenko, W. Zhang, A. Razavi, D. Langley. A highly accurate metadynamics-based Dissociation Free Energy method to calculate protein-protein and protein-ligand binding potencies. Sci. Rep., 2022. 12, 2024. https://doi.org/10.1038/s41598-022-05875-8
M. S. Valdés-Tresanco, M. E. Valdés-Tresanco, P. A. Valiente, E. Moreno. gmx_MMPBSA: A New Tool to Perform End-State Free Energy Calculations with GROMACS. J. Chem. Theory. Comput., 2021, 17, 6281. https://doi.org/10.1021/acs.jctc.1c00645
D. K. Vo, K. T. L. Trinh. Molecular Farming for Immunization: Current Advances and Future Prospects in Plant-Produced Vaccines. Vaccines, 2025, 13, 191. https://doi.org/10.3390/vaccines13020191
B. Pudhuvai, B. Koul, A. K. Mishra. Insights into the world of edible vaccines: From lab to reality. Curr. Res. Biotechnol., 2025, 9, 100290. https://doi.org/10.1016/j.crbiot.2025.100290
A. Mozafari, J. Amani, J. S. Shahsavandi, A. Hatef Salmanian. A Novel Multi-Epitope Edible Vaccine Candidate for Newcastle Disease Virus: In Silico Approach. Iran J. Biotechnol., 2022, 20, e3119. https://doi.org/10.30498/ijb.2022.298822.3119
Y. Devarakonda, A. D. Rajratna, A. Ray, K. Syal. Novel edible multi-epitope vaccine construct against Enterococcus faecalis. Nucleus, 2025, 68, 113. https://doi.org/10.1007/s13237-024-00478-2
C. Campbell, E. Azagra. Edible mRNA vaccine in lettuce chloroplasts. Nat. Rev. Bioeng., 2025, 3, 264. https://doi.org/10.1038/s44222-025-00299-1
M. May. How mRNA is powering a personalized vaccine revolution. Nat. Med., 2024, 30, 2097. https://doi.org/10.1038/d41591-024-00052-y
M. G. Jiji, M. A. Ninan, V. P. Thomas, B. T. Thomas. Edible microalgae: potential candidate for developing edible vaccines. Vegetos, 2024, 37, 788. https://doi.org/10.1007/s42535-023-00636-y
Z. T. Buriev, S. E. Shermatov, D. E. Usmanov, M. K. Mirzakhmedov, K. A. Ubaydullaeva, V. S. Kamburova, B. K. Rakhmanov, M. S. Ayubov, A. N. Abdullaev, J. B. Eshmurzaev, B. O. Mamajonov, A. A. Tulanov, A. A. Ismailova, T. A. Petrova, R. J. Rozumbetov, T. U. Aripova, M. I. Muminov, K. Y. Ermatova, D. A. Dalimova, S. U. Turdikulova, A. Abdukarimov, I. Y. Abdurakhmonov. Tomato-made edible COVID-19 vaccine TOMAVAC induces neutralizing IgGs in the blood sera of mice and humans. Front. Nut., 2024, 10, 1275307. https://doi.org/10.3389/fnut.2023.1275307
A. R. Kumar, S. Ajay, K. Anusha, B. Nair, A. R. Devan, G. Sethi, and L. R. Nath. Emerging Role of Plant-Derived Products in Immunotherapy: Plausible Exposition for HCC Treatment. bioRxiv. Liver Cancer Phytomed., 2025, 2025, 443.
Y. Kang, D. S. Kim, H. Hwang, Y. Kim, Y. Seo, P. Hinterdorfer, K. Ko. Plant-derived recombinant macromolecular PAP-IgG Fc as a novel prostate cancer vaccine candidate eliciting robust immune responses. Transgenic Res., 2025, 34, 1. https://doi.org/10.1007/s11248-025-00433-0
K. Mazur-Włodarczyk, A. Gruszecka-Kosowska, B. Martire, M. F. Mastrototaro, G. Ottaviano, C. Rizzo, M. Sgrulletti, M. M. D. Giudice, V. Moschese. Sustainable Consumption and Production of Edible Plants in the Context of Reaching the EU Climate Neutrality by 2050: A Literature Review. Sustainability, 2024, 16, 10822. https://doi.org/10.3390/su162410822
D. Montin, V. Santilli, A. Beni, G. Costagliola et al. Towards personalized vaccines. Front. Immunol., 2024, 15, 1436108. https://doi.org/10.3389/fimmu.2024.1436108
D. C. Koboldt, L. Ding, E. R. Mardis, R. K. Wilson. Challenges of sequencing human genomes. Brief Bioinform., 2010, 11, 484. https://doi.org/10.1093/bib/bbq016
M. Wang, L. Kurgan, M. Li. A comprehensive assessment and comparison of tools for HLA class I peptide-binding prediction. Brief Bioinform., 2023, 24, bbad150. https://doi.org/10.1093/bib/bbad150
A. L. Kessler, R. F. A. Pieterman, W. A. S. Doff, K. Bezstarosti, R. Bouzid, K. Klarenaar, D. T. S. L. Jansen, R. J. Luijten, J. A. A. Demmers, S. I. Buschow. HLA I immunopeptidome of synthetic long peptide pulsed human dendritic cells for therapeutic vaccine design. NPJ Vacc., 2025, 10, 1. https://doi.org/10.1038/s41541-025-01069-1
G. Visalli, A. Laganà, D. Lo Giudice, S. Calimeri, D. Caccamo, A. Trainito, A. D. Pietro, A. Facciolà. Towards a Future of Personalized Vaccinology: Study on Individual Variables Influencing the Antibody Response to the COVID-19 Vaccine. Vaccines, 2023, 11, 217. https://doi.org/10.3390/vaccines11020217
Ö. Türeci, M. Löwer, B. Schrörs, M. Lang, A. Tadmor, U. Sahin. Challenges towards the realization of individualized cancer vaccines. Nat. Biomed. Eng., 2018, 2, 566. https://doi.org/10.1038/s41551-018-0266-2
P. Navaux, A. Lorenzon, M. Serpa. Challenges in High-Performance Computing. J. Braz. Comput. Soc., 2023, 29, 51. https://doi.org/10.5753/jbcs.2023.2219
S. Duangdangchote, D. S. Seferos, O. Voznyy. Stability and transferability of machine learning force fields for molecular dynamics applications. Digital Discovery, 2024, 3, 2177. https://doi.org/10.1039/d4dd00140k
P. Kovatch, L. Gai, H. M. Cho, E. Fluder, D. Jiang. Optimizing High-Performance Computing Systems for Biomedical Workloads. IEEE International Symposium on Parallel & Distributed Processing, Workshops and Phd Forum: [proceedings] IEEE International Symposium on Parallel & Distributed Processing, Workshops and Phd F. 2020, 2020,183. https://doi.org/10.1109/ipdpsw50202.2020.00040
L. Song, G. Bai, X. S. Liu, B. Li, H. Li. Efficient and accurate KIR and HLA genotyping with massively parallel sequencing data. Genome Res., 2023, 33, 923. https://doi.org/10.1101/gr.277585.122
Q. Zhou, M. Ghezelji, A. Hari, M. K. B. Ford, C. Holley, COVNET Consortium, L. Mirabello, S. Chanock, S. C. Sahinalp, I. Numanagićl. Geny: A Genotyping Tool for Allelic Decomposition of Killer Cell Immunoglobulin-Like Receptor Genes. bioRxiv. 2024. https://doi.org/10.1101/2024.02.27.582413
A. Cox, H. Cevik, H. A. Feldman, L. M. Canaday, N. Lakes, S. N. Waggoner. Targeting natural killer cells to enhance vaccine responses. Trends Pharmacol. Sci., 2021, 42, 789. https://doi.org/10.1016/j.tips.2021.06.004
P. Rettman, M. D. Blunt, R. J. Fulton, A. F. Vallejo, L. Y. Bastidas-Legarda, L. España-Serrano, M. E. Polak, A. Al-Shamkhani, C. Retiere, & S. I. Khakoo. Peptide: MHC-based DNA vaccination strategy to activate natural killer cells by targeting killer cell immunoglobulin-like receptors. J. Immunother. Cancer, 2021, 9, e001912. https://doi.org/10.1136/jitc-2020-001912
A. Conev, R. Fasoulis, S. Hall-Swan, R. Ferreira, L. E. Kavraki. HLAEquity: Examining biases in pan-allele peptide-HLA binding predictors. iScience, 2024, 27, 108613. https://doi.org/10.1016/j.isci.2023.108613
A. Halužan Vasle, M. Moškon. Synthetic biological neural networks: From current implementations to future perspectives. BioSystems, 2024, 237, 105164. https://doi.org/10.1016/j.biosystems.2024.105164
D. Bonnerjee, S. Chakraborty, B. Mukherjee, R. Basu, A. Paul, & S. Bagh. Multicellular artificial neural network-type architectures demonstrate computational problem solving. Nat. Chem. Biol., 2024, 20, 1524. https://doi.org/10.1038/s41589-024-01711-4
P. Stolfi, F. Castiglione, E. Mastrostefano, I. Di. Biase, S. Di Biase, G. Palmieri, & A. Prisco. In-silico evaluation of adenoviral COVID-19 vaccination protocols: Assessment of immunological memory up to 6 months after the third dose. Front. Immunol., 2022, 13, 998262. https://doi.org/10.3389/fimmu.2022.998262
S. Succi, F. Castiglione, M. Bernaschi. Collective Dynamics in the Immune System Response. Phys. Rev. Lett., 1997, 79, 4493. https://doi.org/10.1103/PhysRevLett.79.4493
Y. Jamali. Modeling the Immune System Through Agent-based Modeling: A Mini-review. Immunoregulation, 2024, 6, 3. https://doi.org/10.32598/Immunoregulation.6.1.7
A. Rezvanian, S. M. Vahidipour, A. M. Saghiri. CaAIS: Cellular Automata-Based Artificial Immune System for Dynamic Environments. Algorithms, 2024, 17, 18. https://doi.org/10.3390/a17010018
F. Chiacchio, M. Pennisi, G. Russo, S. Motta, F. Pappalardo. Agent-based modeling of the immune system: NetLogo, a promising framework. Biomed. Res. Int., 2014, 2014, 1. https://doi.org/10.1155/2014/907171
K. S. Corbett, D. K. Edwards, S. R. Leist, O. M. Abiona, S. B. Barnum, R. A. Gillespie, S. Himansu, A. Schäfer, C. T. Ziwawo, A. T. DiPiazza, and K. H. Dinnon. SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness. Nature, 2020, 586, 567. https://doi.org/10.1038/s41586-020-2622-0
U. Sahin, A. Muik, E. Derhovanessian, I. Vogler, L. M. Kranz, M. Vormehr, A. Baum, K. Pascal, J. Quandt, D. Maurus, and S. Brachtendorf. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature, 2020, 586, 594. https://doi.org/10.1038/s41586-020-2814-7
P. Tebas, S. Yang, J. D. Boyer, E. L. Reuschel, A. Patel, A. Christensen-Quick, V. M. Andrade, M. P. Morrow, K. Kraynyak, J. Agnes, M. Purwar, A. Sylvester, J. Pawlicki, E. Gillespie, I. Maricic, F. I. Zaidi, K. Y. Kim, Y. Frase. Dia, D. Frase, P. Pezzoli, L. M. Humeau. Safety and immunogenicity of INO-4800 DNA vaccine against SARS-CoV-2: A preliminary report of an open-label, Phase 1 clinical trial. EClinicalMedicine, 2021, 31, 100689. https://doi.org/10.1016/j.eclinm.2020.100689
C. Y. Wang, K. P. Hwang, H. K. Kuo, W. J. Peng, Y. H. Shen, B. S. Kuo J. H. Huang, H. Liu, Y. H. Ho, F. Lin, S. Ding, Z. Liu, H. T. Wu, C. T. Huang, Y. Lee, M. C. Liu, Y. C. Yang, P. L. Lu, H. C. Tsai, C.H. Lee, T. P. Monath. A multitope SARS-CoV-2 vaccine provides long-lasting B cell and T cell immunity against Delta and Omicron variants. J. Clin. Invest., 2022, 132, e157707. https://doi.org/10.1172/JCI157707
A. S. De Groot, J. Rayner, W. Martin. Modelling the immunogenicity of therapeutic proteins using T cell epitope mapping. Dev Biol (Basel)., 2003, 112, 71.
D. B. Olawade, J. Teke, O. Fapohunda, K. Weerasinghe, S. O. Usman, A. O. Ige, A. C. David-Olawade. Leveraging artificial intelligence in vaccine development: A narrative review. J. Microbiol. Methods, 2024, 224, 106998. https://doi.org/10.1016/j.mimet.2024.106998