Mastering Chirality: Unlocking Bioactive Natural Products with Oxazaborolidines and Oxazaborolidinones Asymmetric Catalysis
Main Article Content
Abstract
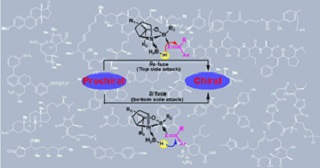
Catalytic asymmetric synthesis has appeared as the preferred method for producing enantiomerically pure compounds, marking significant advancements in recent years. In biological processes, asymmetric catalysis governs the synthesis of chiral compounds, facilitated through the chirality transfer following reactant binding at enzyme active sites. A revolutionary milestone in this area was the discovery of oxazaborolidine chiral catalysts by Corey, Bakshi, and Shibata (CBS catalysts), empowering the enantioselective reduction of achiral ketones. This discovery has had profound implications across industry and academia, establishing oxazaborolidines as pivotal tools for achieving chirality in chemical systems. The application of oxazaborolidines and their derivatives have been extensively explored for enantioselective reductions of various functional groups. While previous review articles focused on specialized functional groups, this review provides an overview (last fifteen years) of practical advancements in the use of borane-oxazaborolidine catalysts for the enantioselective reduction of challenging functional groups such as ketones, ketimines, and oximes. These advancements have facilitated the synthesis of various building blocks for natural products. We also highlighted the potential of oxazaborolidinones as it was remains largely underutilized, presenting an exciting opportunity for future investigations.
Article Details
How to Cite
References
Noyori, R. In Asymmetric Catalysis in Organic Synthesis; John Wiley & Sons: New York, 1994.
H. I. Schlesinger, H. C. Brown, H. R. Hoekstra, L. R. Rapp. Reactions of Diborane with Alkali Metal Hydrides and Their Addition Compounds. New Syntheses of Borohydrides. Sodium and Potassium Borohydrides. J. Am. Chem. Soc. 1953, 75, 199. https://doi.org/10.1021/ja01097a053
A. F. Finholt, A. C. Jr. Bond, H. I. Schlesinger. Acylations of Esters with Esters to Form β-Keto Esters Using Sodium Amide. J. Am. Chem. Soc. 1947, 69, 119. https://doi.org/10.1021/ja01193a033
(a) E. R. Grandbois, S. I. Howard, J. D. Morrison. Asymmetric Synthesis 1983, 2, Academic Press, New York, pp. 71–90. (b) J. W. ApSimon, T. L. Collier, Recent advances in asymmetric synthesis-II. Tetrahedron 1986, 42, 5157. https://doi.org/10.1016/S0040-4020(01)82073-1 (c) H. Haubenstock. Topics in Stereochemistry 1983, 14, Wiley, New York, pp. 231–300. (d) T. Mukaiyama, M. Asami. In Topics in Current Chemistry, Organic Chemistry, 1985, 127, Springer, Berlin, pp. 133-167. (e) A. I. Meyers, P. M. Kendall. Synthesis via oxazolines. VII. Asymmetric reduction of ketones with chiral hydride reagents. Tetrahedron Lett. 1974, 15, 1337. https://doi.org/10.1016/S0040-4039(01)82482-5 (f) M. F. Grundon, D. G. McCleery, J. W. Wilson. ibid. 1976, 17, 295.
(a) S. Yamaguchi, H. S. Mosher. Asymmetric reductions with chiral reagents from lithium aluminum hydride and (+)-(2S,3R)-4-dimethylamino-3-methyl-1,2-diphenyl-2-butanol. J. Org. Chem. 1973, 38, 1870. https://doi.org/10.1021/jo00950a020 (b) R. S. Brinkmeyer, V. M. Kapoor. Asymmetric reduction. Reduction of acetylenic ketones with chiral hydride agent. J. Am. Chem. Soc. 1977, 99, 8339. https://doi.org/10.1021/ja00467a047 (c) N. Cohen, R. J. Lopresti, C. Neukom, G. Saucy. Asymmetric reductions of α, β-acetylenic ketones and acetophenone using lithium aluminum hydride complexed with optically active 1,3-amino alcohols. J. Org. Chem. 1980, 45, 582. https://doi.org/10.1021/jo01292a006
(a) A. Hirao, S. Itsuno, S. Nakahama, N. Yamazaki. Asymmetric reduction of aromatic ketones with chiral alkoxy-amineborane complexes. J. Chem. Soc. Chem. Commun. 1981, 315. https://doi.org/10.1039/C39810000315 (b) S. Itsuno, A. Hirao, S. Nakahama, N. Yamazaki. Asymmetric synthesis using chirally modified borohydrides. Part 1. Enantioselective reduction of aromatic ketones with the reagent prepared from borane and (S)-valinol. J. Chem. Soc. Perkin Trans. 1 1983, 1673. https://doi.org/10.1039/P19830001673
S. Itsuno, M. Nakano, K. Miyazaki, V. Masuda, K. Ito, A. Hirao, S. Nakahama. Asymmetric synthesis using chirally modified borohydrides. Part 3. Enantioselective reduction of ketones and oxime ethers with reagents prepared from borane and chiral amino alcohols. J. Chem. Soc. Perkin Trans. 11985, 2039. https://doi.org/10.1039/P19850002039
(a) R. Noyori, I. Tomino, Y. Tanimoto, M. Nishizawa. Asymmetric synthesis via axially dissymmetric molecules. 6. Rational designing of efficient chiral reducing agents. Highly enantioselective reduction of aromatic ketones by binaphthol-modified lithium aluminum hydride reagents. J. Am. Chem. Soc. 1984, 106, 6709. https://doi.org/10.1021/ja00334a041 (b) R. Noyori, I. Tomino, M. Yamada, M. Nishizawa. Asymmetric synthesis via axially dissymmetric molecules. 7. Synthetic applications of the enantioselective reduction by binaphthol-modified lithium aluminum hydride reagents. J. Am. Chem. Soc. 1984, 106, 6717. https://doi.org/10.1021/ja00334a042
(a) E. J. Corey, R. K. Bakshi, S. Shibata. Highly enantioselective borane reduction of ketones catalyzed by chiral oxazaborolidines. Mechanism and synthetic implications. J. Am. Chem. Soc. 1987, 109, 5551. https://doi.org/10.1021/ja00252a056 (b) E. J. Corey, T. Shibata, T. W. Lee. Asymmetric Diels−Alder Reactions Catalyzed by a Triflic Acid Activated Chiral Oxazaborolidine. J. Am. Chem. Soc. 2002, 124, 3808. https://doi.org/10.1021/ja025848x
E. J. Corey, C. J. Helal. Reduction of Carbonyl Compounds with Chiral Oxazaborolidine Catalysts: A New Paradigm for Enantioselective Catalysis and a Powerful New Synthetic Method. Angew. Chem. Int. Ed. 1998, 37, 1986. https://doi.org/10.1002/(SICI)1521-3773(19980817)37:15<1986::AID-ANIE1986>3.0.CO;2-Z
C. Puigjaner, A. V. Ferran, A. Moyano, M. A. Pericàs, A. Riera. A New Family of Modular Chiral Ligands for the Catalytic Enantioselective Reduction of Prochiral Ketones. J. Org. Chem. 1999, 64, 7902. https://doi.org/10.1021/jo990942q
(a) S. Itsuno, M. Nakano, K. Miyazaki, H. Masuda, K. Ito, A. Hirao, S. Nakahama. Asymmetric synthesis using chirally modified borohydrides. Part 3. Enantioselective reduction of ketones and oxime ethers with reagents prepared from borane and chiral amino alcohols. J. Chem. Soc., Perkin Trans. I 1985, 2039. https://doi.org/10.1039/P19850002039 (b) S. Itsuno, Y. Sakurai, K. Ito, A. Hirao, S. Nakahama. Catalytic Behavior of Optically Active Amino Alcohol–Borane Complex in the Enantioselective Reduction of Acetophenone Oxime O-Alkyl Ethers. Bull. Chem. Soc. Jpn. 1987, 60, 395. https://doi.org/10.1246/bcsj.60.395 (c) S. Itsuno, Y. Sakurai, K. Shimizu, K. Ito. Asymmetric reduction of ketoxime O-alkyl ethers with chirally modified NaBH4–ZrCl4. J. Chem. Soc., Perkin Trans. 1 1990, 1859. https://doi.org/10.1039/P19900001859 (d) E. J. Corey, R. K. Bakshi, S. Shibata. Highly enantioselective borane reduction of ketones catalyzed by chiral oxazaborolidines. Mechanism and synthetic implications. J. Am. Chem. Soc. 1987, 109, 5551. https://doi.org/10.1021/ja00252a056 (e) E. J. Corey, R. K. Bakshi, S. Shibata, C. –P. Chen, V. K. Singh. A stable and easily prepared catalyst for the enantioselective reduction of ketones. Applications to multistep syntheses. J. Am. Chem. Soc. 1987, 109, 7925. https://doi.org/10.1021/ja00259a075 (f) R. Berenguer, J. Garcia, J. Vilarrasa. Enantioselective reduction of ketones catalysed by 1,3,2-oxazaborolidines prepared from phenylglycine. Tetrahedron: Asymmetry 1994, 5, 165. https://doi.org/10.1016/S0957-4166(00)86163-7 (g) G. J. Quallich, J. F. Blake, T. M. Woodall. A combined synthetic and ab initio study of chiral oxazaborolidines structure and enantioselectivity relationships. J. Am. Chem. Soc. 1994, 116, 8516. https://doi.org/10.1021/ja00098a012 (h) N. N. Joshi, M. Srebnik, H. C. Brown. Chiral Oxazaborolidines as Catalysts for the Enantioselective Addition of Diethylzinc to Aldehydes. Tetrahedron Lett. 1989, 30, 5551. https://doi.org/10.1016/S0040-4039(01)93797-9 (h) Y. Hong, Y. Gao, X. Nie, C. M. Zepp. cis-1-amino-2-indanol in asymmetric synthesis. Part I. A practical catalyst system for the enantioselective borane reduction of aromatic ketones. Tetrahedron Lett. 1994, 35, 6631. https://doi.org/10.1016/S0040-4039(00)73453-8
(a) A. Hirao, S. Itsuno, S. Nakahama, N. Yamazaki. Asymmetric reduction of aromatic ketones with chiral alkoxy-amineborane complexes. J. Chem. Soc., Chem. Commun. 1981, 315. https://doi.org/10.1039/C39810000315 (b) S. Itsuno, M. Nakano, K. Miyazaki, H. Masuda, K. Ito, A. Hirao, S. Nakahama. Asymmetric synthesis using chirally modified borohydrides. Part 3. Enantioselective reduction of ketones and oxime ethers with reagents prepared from borane and chiral amino alcohols. J. Chem. Soc., Perkin Trans. 11985, 2039. https://doi.org/10.1039/P19850002039
(a) E. J. Corey, R. K. Bakshi, S. Shibata. Highly enantioselective borane reduction of ketones catalyzed by chiral oxazaborolidines. Mechanism and synthetic implications. J. Am. Chem. Soc. 1987, 109, 5551. https://doi.org/10.1021/ja00252a056 (b) E. J. Corey, R. K. Bakshi, S. Shibata, C.-P. Chen, V. K. Singh. A stable and easily prepared catalyst for the enantioselective reduction of ketones. Applications to multistep syntheses. J. Am. Chem. Soc. 1987, 109, 7925. https://doi.org/10.1021/ja00259a075 (c) E. J. Corey, M. Azimioara, S. Sarshar. X-Ray crystal structure of a chiral oxazaborolidine catalyst for enantioselective carbonyl reduction. Tetrahedron Lett. 1992, 33, 3429. https://doi.org/10.1016/S0040-4039(00)92654-6
For review, see: (a) D. P. Schwinger, T. Bach. Chiral 1,3,2-oxazaborolidine catalysts for enantioselective photochemical reactions. Acc. Chem. Res. 2020, 53, 1933. https://doi.org/10.1021/acs.accounts.0c00379 (b) Y. Kawanami, R. C. Yanagita. Practical Enantioselective Reduction of Ketones Using Oxazaborolidine Catalysts Generated In Situ from Chiral Lactam Alcohols. Molecules 2018, 23, 1. https://doi.org/10.3390/molecules23102408 (c) S. Y. Shim, D. H.Ryu. Enantioselective Carbonyl 1,2- or 1,4-Addition Reactions of Nucleophilic Silyl and Diazo Compounds Catalyzed by the Chiral Oxazaborolidinium Ion. Acc. Chem. Res. 2019, 52, 2349. https://doi.org/10.1021/acs.accounts.9b00279 (d) B. T. Cho. Recent advances in the synthetic applications of the oxazaborolidine-mediated asymmetric reduction. Tetrahedron, 2006, 62, 7621. https://doi.org/10.1016/j.tet.2006.05.036 (e) B. T. Cho. Boronic Acids: Preparation and Applications in Organic Synthesis; D. G. Hall, Ed.; Wiley- VCH: Weinheim, 2005; Chapter 11, p 411. (f) S. Itsuno. Comprehensive Asymmetric Catalysts; E. N. Jacobsen, A. Pfaltz, H. Yamamoto, Eds.; Springer: New York, NY, 1999; Vol. 1, p 289. (g) E. J. Corey, C. J. Helal. Reduction of Carbonyl Compounds with Chiral Oxazaborolidine Catalysts: A New Paradigm for Enantioselective Catalysis and a Powerful New Synthetic Method. Angew. Chem., Int. Ed. 1998, 37, 1986. https://doi.org/10.1002/(SICI)1521-3773(19980817)37:15<1986::AID-ANIE1986>3.0.CO;2-Z (g) L. Deloux, M. Srebnik. Asymmetric boron-catalyzed reactions. Chem. Rev. 1993, 93, 763. https://doi.org/10.1021/cr00018a007 (h) V. K. Singh. Practical and Useful Methods for the Enantioselective Reduction of Unsymmetrical Ketones. Synthesis 1992, 605. https://doi.org/10.1055/s-1992-26174 (i) S. Wallbaum, J. Martens. Asymmetric syntheses with chiral oxazaborolidines. Tetrahedron: Asymmetry 1992, 3, 1475. https://doi.org/10.1016/S0957-4166(00)86044-9
(a) K. Z. Łaczkowski, Z. Czyznikowska, R. Zales´ny, A. Baranowska-Łaczkowska. The B–H–B bridging interaction in B-substituted oxazaborolidine–borane complexes: a theoretical study. Struct Chem., 2013, 24, 1485. https://doi.org/10.1007/s11224-012-0178-9 (b) H.S. Kettouche, A. Djerourou. Insights into the origin of selectivity for [2+2] cycloaddition step reaction involved in the mechanism of enantioselective reduction of ketones with borane catalyzed by a B-methoxy oxazaborolidine catalyst derived from (–)-β-pinene: an HMDFT and combined topological ELF, NCI and QTAIM study. Theor. Chem. Acc. 2021, 140, 150. https://doi.org/10.1007/s00214-021-02848-4 (c) Z. Lachtar, A. K. Nacereddine, A. Djerourou. Understanding the origin of the enantioselectivity and the mechanism of the asymmetric reduction of ketimine generated from acetophenone with oxazaborolidine catalyst. Struct. Chem. 2020, 31, 253. https://doi.org/10.1007/s11224-019-01400-2
M. Masui, T. Shioiri. A Practical Method for Asymmetric Borane Reduction of Prochiral Ketones Using Chiral Amino Alcohols and Trimethyl Borate. Synlett 1997, 3, 273. https://doi.org/10.1055/s-1997-779
(a) K. M. Reddy, E. Bhimireddy, B. Thirupathi, S.Breitler, S. Yu, E. J. Corey. Cationic chiral fluorinated oxazaborolidines. More potent second- generation catalyst for highly enanatioselective cycloaddition reactions. J. Am. Chem. Soc. 2016, 138, 2443. https://doi.org/10.1021/jacs.6b00100 (b) B. Thirupathi, S. Breitler, K. M. Reddy, E. J. Corey. Acceleration of Enantioselective Cycloadditions Catalyzed by Second-Generation Chiral Oxazaborolidinium Triflimidates by Biscoordinating Lewis Acids. J. Am. Chem. Soc. 2016, 138, 10842. https://doi.org/10.1021/jacs.6b08018
J. M. Wahl, M. L. Conner, M. K. Brown. Synthesis of (−)-Hebelophyllene E: An Entry to Geminal Dimethyl-Cyclobutanes by [2+2] Cycloaddition of Alkenes and Allenoates. Angew. Chem. Int. Ed. 2018, 57, 4647. https://doi.org/10.1002/anie.201801110
A. Moscona. Neuraminidase Inhibitors for Influenza. New Engl. J. Med. 2005, 353, 1363. https://doi.org/10.1056/NEJMra050740
Y. Y. Yeung, S. Hong, E. J. Corey. A Short Enantioselective Pathway for the Synthesis of the Anti-Influenza Neuramidase Inhibitor Oseltamivir from 1,3-Butadiene and Acrylic Acid. J. Am. Chem. Soc. 2006, 128, 6310. https://doi.org/10.1021/ja0616433
(a) J. Matsuo, T. Kozai, O. Nishikawa, Y. Hattori, H. Ishibashi. Oxazaborolidine-Catalyzed Enantioselective Reduction of α-Methylene Ketones to Allylic Alcohols. J. Org. Chem. 2008, 73, 6902. https://doi.org/10.1021/jo8011013 (b) T. Yıldız. An oxazaborolidine-based catalytic method for the asymmetric synthesis of chiral allylic alcohols. Tetrahedron: Asymmetry, 2015, 26, 497. https://doi.org/10.1016/j.tetasy.2015.03.008
(a) S. Goodin, M. P. Kane, E. H. Rubin. Epothilones: Mechanism of Action and Biologic Activity. J. Clin. Oncol. 2004, 22, p. 2015. https://doi.org/10.1200/JCO.2004.12.0 (b) D. M. Bollag, P. A. McQueney, J. Zhu, O. Hensens, L. Koupal, J. Liesch, M. Goetz, E. Lazarides, C. M. Woods. Epothilones, a New Class of Microtubule-stabilizing Agents with a Taxol-like Mechanism of Action, Cancer Res. 1995, 55, 2325. (c) K. C. Nicolaou, F. Roschangar, D. Vourloumis. Chemical Biology of Epothilones. Angew. Chem., Int. Ed. 1998, 37, 2014. https://doi.org/10.1002/(SICI)1521-3773(19980817)37:15<2014::AID-ANIE2014>3.0.CO;2-2 (d) C. R. Harris, S. J. Danishefsky. Complex Target-Oriented Synthesis in the Drug Discovery Process: A Case History in the dEpoB Series. J. Org. Chem. 1999, 64, 8434. https://doi.org/10.1021/jo991006d (e) K. H. Altmann, M. Wartmann, T. O’Reilly. Epothilones and related structures- a new class of microtubule inhibitors with potent in vivo antitumor activity. Biochim. Biophys. Acta, 2000, 1470, M79. https://doi.org/10.1016/s0304-419x(00)00009-3 (f) K. C. Nicolaou, A. Ritzen, K. Namoto. Recent developments in the chemistry, biology and medicine of the epothilonesDedicated to Drs Margaret Harris and Muriel Hall of Bedford College, London, for their dedicated and inspiring teachings and research contributions in celebration of their retirement. Chem. Commun. 2001, 1523. https://doi.org/10.1039/B104949F (g) M. Wartmann, K. H. Altmann. The biology and medicinal chemistry of epothilones. Curr. Med. Chem.: Anti-Cancer Agents2002, 2, 123. https://doi.org/10.2174/1568011023354489 (h) E. B. Watkins, A. G. Chittiboyina, M. A. Avery. Recent Developments in the Syntheses of the Epothilones and Related Analogues. Eur. J. Org. Chem. 2006, 4071. https://doi.org/10.1002/ejoc.200600149 (i) E. B. Watkins, A. G. Chittiboyina, J.-C. Jung, M. A. Avery. The Epothilones and Related Analogues-A Review of Their Syntheses and Anti-Cancer Activities. Curr. Pharm. Des. 2005, 11, 1615. https://doi.org/10.2174/1381612053764742 (j) F. Feyen, F. Cachoux, J. Gertsch, M. Wartmann, K.-H. Altmann. Epothilones as Lead Structures for the Synthesis-Based Discovery of New Chemotypes for Microtubule Stabilization. Acc. Chem. Res. 2008, 41, 21. https://doi.org/10.1021/ar700157x (k) R. J. Kowalski, P. Giannakakou, E. Hamel. Activities of the Microtubule-stabilizing Agents Epothilones A and B with Purified Tubulin and in Cells Resistant to Paclitaxel (Taxol®). J. Biol. Chem.1997, 272, 2534. https://doi.org/10.1074/jbc.272.4.2534 (l) W. M. Smit, J. Sufliarsky, S. Spanik, M. Wagnerov, S. Kaye, A. M. Oza, M. Gore, K. J. Williams, A. Johri, W. W. T. B. Huinink. J. Clin. Oncol.2005, 23, 16S. (m) S. McMeekin, R. Patel, C. Verschraegan, P. Celano, J. Burke, S. Plaxe, P. Ghatage, M. Giurescu, C. Stredder, Y. Wang. In 33rd Congress of the European Society for Medical Oncology, 2008. (n) C. Sessa, A. Perotti, A. Llado, S. Cresta, G. Capri, M. Voi, S. Marsoni, I. Corradino, L. Gianni. Ann. Oncol. 2007, 18, 1548. (o) A. Stopeck, S. Moulder, S. Jones, J. Cohen, M. McDowell, G. Cropp, Z. Zhong, S. Wells, A. Hannah, H. Burris. J. Clin. Oncol. 2007, 25, 2571. (p) S. Bhushan, C. M. Walko, Ann. Pharmacother. 2008, 42, 1252.
E. A. Reiff, S. K. Nair, J. T. Henri, J. F. Greiner, B. S. Reddy, R. Chakrasali, S. A. David, T. L. Chiu, E. A. Amin, R. H. Himes, D. G. V. Velde, G. I. Georg. Total Synthesis and Evaluation of C26-Hydroxyepothilone D Derivatives for Photoaffinity Labeling of β-Tubulin. J. Org. Chem.2010, 75, 86. https://doi.org/10.1021/jo901752v
(a) A. S. Pilcher, P. DeShong. Improved protocols for the selective deprotection of trialkylsilyl ethers using fluorosilicic acid. J. Org. Chem.1993, 58, 5130. https://doi.org/10.1021/jo00071a023 (b) D. Schinzer, A. Bauer, O. M. Bohm, A. Limberg, M. Cordes, Total Synthesis of (−)-Epothilone A. Chem. Eur. J. 1999, 5, 2483. https://doi.org/10.1002/(SICI)1521-3765(19990903)5:9<2483::AID-CHEM2483>3.0.CO;2-N (c) K. C. Nicolaou, S. Ninkovic, F. Sarabia, D. Vourloumis, Y. He, H. Vallberg, M. R. V. Finlay, Z. Yang. Total Syntheses of Epothilones A and B via a Macrolactonization-Based Strategy. J. Am. Chem. Soc. 1997, 119, 7974. https://doi.org/10.1021/ja971110h (d) K. C. Nicolaou, D. Hepworth, N. P. King, M. R. V. Finlay, R. Scarpelli, M. M. A. Pereira, B. Bollbuck, A. Bigot, B. Werschkun, N. Winssinger. Total Synthesis of 16-Desmethylepothilone B, Epothilone B10, Epothilone F, and Related Side Chain Modified Epothilone B Analogues. Chem. Eur. J. 2000, 6, 2783. https://doi.org/10.1002/1521-3765(20000804)6:15<2783::AID-CHEM2783>3.0.CO;2-B (e) K. C. Nicolaou, D. Hepworth, M. R. V. Finlay, N. Paul King, B. Werschkun, A. Bigot. Synthesis of 16-desmethylepothilone B: improved methodology for the rapid, highly selective and convergent construction of epothilone B and analogues. Chem. Commun. 1999, 519. https://doi.org/10.1039/A809954E (f) J. P. Genet, V. Ratovelomanana-Vidal, M. C. Cano de Andrade, X. Pfister, P. Guerreiro, J. Y. Lenoir. Practical asymmetric hydrogenation of β-keto esters at atmospheric pressure using chiral Ru (II) catalysts. Tetrahedron Lett. 1995, 36, 4801. https://doi.org/10.1016/0040-4039(95)00873-B (g) D. F. Taber, L. J. Silverberg. Enantioselective reduction of β-keto esters. Tetrahedron Lett. 1991, 32, 4227. https://doi.org/10.1016/S0040-4039(00)92134-8 (h) L. A. Paquette, R. A. Jr. Galemmo, J. C. Caille, R. S. Valpey. Synthetic studies relating to the structure of senoxydene. A sequential annulation approach to angular triquinane construction capable of varied tetramethyl substitution patterns. J. Org. Chem. 1986, 51, 686. https://doi.org/10.1021/jo00355a019 (i) J. Mulzer, A. Mantoulidis, E. Oehler. Total Syntheses of Epothilones B and D. J. Org. Chem.2000, 65, 7456. https://doi.org/10.1021/jo0007480 (j) K. C. Nicolaou, M. R. V. Finlay, S. Ninkovic, F. Sarabia. Total synthesis of 26-hydroxy-epothilone B and related analogs via a macrolactonization based strategy. Tetrahedron 1998, 54, 7127. https://doi.org/10.1016/S0040-4020(98)00352-4
T. A. Rano, G. H. Kuo. Improved Asymmetric Synthesis of 3,4-Dihydro-2-[3-(1,1,2,2-tetrafluoroethoxy)phenyl]-5-[3-(trifluoromethoxy)phenyl]-α-(trifluoromethyl)-1(2H)-quinolineethanol, a Potent Cholesteryl Ester Transfer Protein Inhibitor. Org. Lett. 2009, 11, 2812 https://doi.org/10.1021/ol900639j and the references cited therein.
(a) E. Canales, E. J. Corey. Highly Enantioselective [4 + 2] Cycloaddition Reactions Catalyzed by a Chiral N-Methyl-oxazaborolidinium Cation. Org. Lett. 2008, 10, 3271. https://doi.org/10.1021/ol8011502 (b) Q.-Y. Hu, G. Zhou, E. J. Corey, Application of Chiral Cationic Catalysts to Several Classical Syntheses of Racemic Natural Products Transforms Them into Highly Enantioselective Pathways. J. Am. Chem. Soc. 2004, 126, 13708 https://doi.org/10.1021/ja046154m and the references cited therein.
S. H. von Reuss, W. A. Kӧnig. Corsifurans A–C, 2-arylbenzofurans of presumed stilbenoid origin from Corsinia coriandrina (Hepaticae). Phytochemistry 2004, 65, 3113. https://doi.org/10.1016/j.phytochem.2004.10.002
B. D. Gates, P. Dalidowicz, A. Tebben, S. Wang, J. S. Sweton. Mechanistic aspects and synthetic applications of the electrochemical and iodobenzene bistrifluoroacetate oxidative 1,3-cycloadditions of phenols and electron-rich styrene derivatives. J. Org. Chem. 1992, 57, 2135. https://doi.org/10.1021/jo00033a040
(a) M. P. Krzemiński. 13.7. Asymmetric Reduction of Acetophenone with Borane Catalyzed by B -Methoxy-oxazaborolidine. 2016, 803. https://doi.org/10.1039/9781849739634-00803 (b) H. Adams, N. J. Gilmore, S. Jones, M. P. Muldowney, S. H. von Reuss, R. Vemula. Asymmetric Synthesis of Corsifuran A by an Enantioselective Oxazaborolidine Reduction. Org. Lett. 2008, 10, 1457. https://doi.org/10.1021/ol800239q (c) Y. Kawanami, K. Hoshino, W. Tsunoi. Enantioselective reduction of trifluoromethyl ketones using an oxazaborolidine catalyst generated in situ from a chiral lactam alcohol. Tetrahedron: Asymmetry 2011, 22, 1464. https://doi.org/10.1016/j.tetasy.2011.08.006 (d) Y. Harauchi, C. Takakura, T. Furumoto, R.C. Yanagita, Y. Kawanami. Effect of BF3 on the enantioselective reduction of trifluoromethyl ketones using a chiral lactam alcohol with borane. Tetrahedron: Asymmetry, 2015, 26, 333. https://doi.org/10.1016/j.tetasy.2015.02.011
(a) E. J. Corey, X. Cheng, K. A. Cimprich, S. Sarshar. Remarkably effective and simple syntheses of enantiomerically pure secondary carbinols from achiral ketones. Tetrahedron Lett. 1991, 32, 6835. https://doi.org/10.1016/0040-4039(91)80419-7 (b) E. J.; Corey, J. O. Link, R. K. Bakshi. A mechanistic and structural analysis of the basis for high enantioselectivity in the oxazaborolidine-catalyzed reduction of trihalomethyl ketones by catecholborane. Tetrahedron Lett. 1992, 33, 7107. https://doi.org/10.1016/S0040-4039(00)60848-1 (c) V. Stepanenko, M. D. Jesus, W. Correa, I. Guzman, C. Vazquez, W. Cruz, M. Ortiz-Marciales, C. L. Barnes. Enantioselective reduction of prochiral ketones using spiroborate esters as catalysts. Tetrahedron Lett. 2007, 48, 5799. https://doi.org/10.1016/j.tetlet.2007.06.086 (d) T. Korenaga, K. Nomura, K. Onoue, T. Sakai. Rational electronic tuning of CBS catalyst for highly enantioselective boranereduction of trifluoroacetophenone. Chem. Commun. 2010, 46, 8624. https://doi.org/10.1039/C0CC03706K
C. H. V. A. Sasikala, P. R. Padi, V. Sunkara, P. Ramayya, P. K. Dubey, V. B. R. Uppala, C. Praveen. An Improved and Scalable Process for the Synthesis of Ezetimibe: An Antihypercholesterolemia Drug. Org. Process Res. Dev. 2009, 13, 907. https://doi.org/10.1021/op900039z
S. Tamura, S. Doke, N. Murakami. Total synthesis of peumusolide A, NES non-antagonistic inhibitor for nuclear export of MEK. Tetrahedron 2010, 66, 8476. https://doi.org/10.1016/j.tet.2010.08.025
S. Asano, T. Nakamura, M. Adachi, M. Yoshida, M. Yanagida, E. Nishida. CRM1 is responsible for intracellular transport mediated by the nuclear export signal. Nature 1997, 390, 308. https://doi.org/10.1038/36894
S. Tamura, Y. Hattori, M. Kaneko, N. Shimizu, S. Tanimura, M. Khono, N. Murakami. Peumusolide A, unprecedented NES non-antagonistic inhibitor for nuclear export of MEK. Tetrahedron Lett. 2010, 51, 1678. https://doi.org/10.1016/j.tetlet.2010.01.068
(a) C.-Y. Chen, C.-H. Chen, Y.-C. Lo, B.-N. Wu, H.-M. Wang, W.-L. Lo, C.-M. Yen, R.-J. Lin. Anticancer Activity of Isoobtusilactone A from Cinnamomum kotoense: Involvement of Apoptosis, Cell-Cycle Dysregulation, Mitochondria Regulation, and Reactive Oxygen Species. J. Nat. Prod. 2008, 71, 933. https://doi.org/10.1021/np070620e (b) P.-L. Kuo, C.-Y. Chen, T.-F. Tzeng, C.-C. Lin, Y.-L. Hsu. Involvement of reactive oxygen species/c-Jun NH2-terminal kinase pathway in kotomolide A induces apoptosis in human breast cancer cells. Toxicol. Appl. Pharmacol. 2008, 229, 215. https://doi.org/10.1016/j.taap.2008.01.034
S. Hong, E. J. Corey. Enantioselective Syntheses of Georgyone, Arborone, and Structural Relatives. Relevance to the Molecular-Level Understanding of Olfaction. J. Am. Chem. Soc. 2006, 128, 1346. https://doi.org/10.1021/ja057483x
(a) Y. Shimoda, H. Yamamoto. New oxazaborolidine catalyst for the Diels-Alder Reaction, Synthesis2017, 49, 175. https://doi.org/10.1055/s-0036-1588082 (b) S. –L. Zhang, Y. Lu, Y.-H. Li, K.-Y. Wang, J. -H. Chen, Z. Yang. Catalytic and Enantioselective Diels–Alder Reactions of (E)-4-Oxopent-2-enoates. Org. Lett. 2017, 19, 3986. https://doi.org/10.1021/acs.orglett.7b01692
B. T. Cho. Aldrichimica Acta 2002, 35, 3.
J. Xiao, Z. Z. Wong, Y. P. Lu, T. P. Loha. Hexahydropyrrolo[2,3-b]indoles: A New Class of Structurally Rigid Tricyclic Skeleton for Oxazaborolidine-Catalyzed Asymmetric Borane Reduction. Adv. Synth. Catal. 2010, 352, 1107. https://doi.org/10.1002/adsc.200900908
J. H. Yune, F. Quignard, K. Molvinger. Enantioselectivity Induced by Oxazaborolidine Supported on Mesoporous Silica or by Its Analog in Homogeneous Phase. Molecules 2010, 15, 3643. https://doi.org/10.3390/molecules15053643 and cited therein.
H. S. Kettouche, A. H. Djerourou. A New Method for the Preparation of Oxazaborolidine Catalyst In Situ Using 1,2-Aminoalcohol, Sodium Borohydride, and Diiodomethane for the Asymmetric Reduction of Prochiral Ketones and N-Substituted Imines. The Open Catalysis Journal, 2009, 2, 96. https://doi.org/10.2174/1876214X00902010096
D. Liu, S. Hong, E. J. Corey. Enantioselective synthesis of bridged-or fused-ring bicyclic ketones by a catalytic asymmetric Michael addition pathway. J. Am. Chem. Soc. 2006, 128, 8160. https://doi.org/10.1021/ja063332y
R. K. Jr. Boeckman, J. E. Pero, D. J. Boehmler. Toward the Development of a General Chiral Auxiliary. Enantioselective Alkylation and a New Catalytic Asymmetric Addition of Silyloxyfurans: Application to a Total Synthesis of (−)-Rasfonin. J. Am. Chem. Soc. 2006, 128, 11032. https://doi.org/10.1021/ja063532%2B
(a) I. U. Kutama, S. Jones. Enantioselective Desymmetrization of Glutarimides Catalyzed by Oxazaborolidines Derived from cis-1-Amino-indan-2-ol. J. Org. Chem. 2015, 80, 1468. https://doi.org/10.1021/acs.joc.5b02177 (b) B. J. Marsh, H. Adams, M. D. Barker, I. U. Kutama, S. Jones. Enantioselective catalytic desymmetrization of maleimides by temporary removal of an internal mirror plane and stereoablative over-reduction: synthesis of (R)-pyrrolam A. Org. Lett. 2014, 16, 3780. https://doi.org/10.1021/ol5016702
E. H. E. Farrar, M. N. Grayson. Noncovalent Interactions in the Oxazaborolidine-Catalyzed Enantioselective Mukaiyama Aldol. J. Org. Chem. 2022, 87, 15, 10054. https://doi.org/10.1021/acs.joc.2c01039
L. J. Drummond, A. Sutherland. Asymmetric synthesis of allylic secondary alcohols: a new general approach for the preparation of α-amino acids. Tetrahedron 2010, 66, 5349. https://doi.org/10.1016/j.tet.2010.05.066
T. F. Yang, C. H. Shen, C. T. Hsu, L. H. Chen, C. H. Chuang. Selective reaction of camphor-derived exo-formyl [2.2.1]bicyclic carbinol with alkyl primary amines: application to the preparation of new chiral catalysts for asymmetric reduction of aryl ketones. Tetrahedron 2010, 66, 8734. https://doi.org/10.1016/j.tet.2010.09.008
(a) M. P. Krzeminski, M. Zaidlewicz. Asymmetric reduction of ketoxime derivatives and N-alkylketimines with borane–oxazaborolidine adducts. Tetrahedron: Asymmetry 2003, 14, 1463. https://doi.org/10.1016/S0957-4166(03)00314-8 (b) M. Zaidlewicz, A. Tafelska-Kaczmarek, A. Prewysz-Kwinto. Enantioselective reduction of benzofuryl halomethyl ketones: asymmetric synthesis of (R)-bufuralol. Tetrahedron: Asymmetry 2005, 16, 3205. https://doi.org/10.1016/j.tetasy.2005.08.012 (c) M. J. Bosiak, M. P. Krzeminski, P. Jaisankar, M. Zaidlewicz. Asymmetric synthesis of N-1-(heteroaryl)ethyl-N-hydroxyureas. Tetrahedron: Asymmetry2008, 19, 956. https://doi.org/10.1016/j.tetasy.2008.03.026 (d) K. Z. Łączkowski, M. M. Pakulski, M. P. Krzemiński, P. Jaisankar, M. Zaidlewicz. Asymmetric synthesis of N-substituted N-hydroxyureas. Tetrahedron: Asymmetry 2008, 19, 788. https://doi.org/10.1016/j.tetasy.2008.03.008 (e) M. M. Pakulski, S. K. Mahato, M. J. Bosiak, M. P. Krzeminski, M. Zaidlewicz. Enantioselective reduction of ketoxime ethers with borane–oxazaborolidines and synthesis of the key intermediate leading to (S)-rivastigmine. Tetrahedron: Asymmetry 2012, 23, 716. https://doi.org/10.1016/j.tetasy.2012.05.008
J. Kaldun, A. Krimalowski, M. Breuning. Enantioselective borane reduction of ketones catalysed by triclinic 1,3,2-oxazaborolidines. Tetrahedron Lett. 2016, 57, 2492. https://doi.org/10.1016/j.tetlet.2016.04.091
H. S. Kettouche. A DFT study on the reaction mechanism of enantioselective reduction of ketones with borane catalyzed by a B-methoxy-oxazaborolidine catalyst derived from (-)-β-pinene. J Mol. Model. 2020, 26:27. https://doi.org/10.1007/s00894-019-4276-0
X. Liang, M. Yoo, T. Schempp, S. Maejima, M. J. Krische. Ruthenium-Catalyzed Butadiene-Mediated Crotylation and Oxazaborolidine-Catalyzed Vinylogous Mukaiyama Aldol Reaction for The Synthesis of C1–C19 and C23–C35 of Neaumycin B. Angew. Chem. Int. Ed. 2022, 61, e20221478. https://doi.org/10.1002/anie.202214786
W. Disadee, S. Ruchirawat. Oxazaborolidine-catalyzed reductive parallel kinetic resolution of ketones from β-nitro-azabicycles for the synthesis of chiral hypoestestatins 1, 2. Org. Biomol. Chem., 2021, 19, 8794. https://doi.org/10.1039/D1OB01608C
A. Pinaka, D. Dimotikali, B. Chankvetadze, K. Papadopoulos, G. C. Vougioukalakis. Catalytic Asymmetric Reduction of Prochiral Ketones with Chiral β-Amino Alcohol N-Boranes and the Corresponding Tris(oxazaborolidine)borazines. Synlett 2013, 24, 2401. https://doi.org/10.1055/s-0033-1339943
M. V. Reddy, P. Shyamala, A. K. Sharma. An efficient, concise, and scalable synthesis of Izenamide A and B via asymmetric reduction of γ-amino β-keto ester using 2-methyl-CBS-oxazaborolidine catalysts. Results in Chemistry, 2023, 5, 100756. https://doi.org/10.1016/j.rechem.2022.100756
S. Kiyooka, M. A. Hema. A chiral oxazaborolidinone-promoted aldol reaction with a silyl ketene acetal from ethyl 1,3-dithiolane-2-carboxylate. Synthesis of acetate aldols in high enantiomeric purity. Tetrahedron: Asymmetry, 1996, 7, 2181. https://doi.org/10.1016/0957-4166(96)00265-0
K. Komura, S. Itsuno. Asymmetric Aldol Polymerization of Bis(trimethylsilylketene thioacetal) and Dialdehyde. Chem. Lett. 2001, 730. https://doi.org/10.1246/cl.2001.730
T. Harada, T. Egusa, Y. Igarashi, M. Kinugasa, A. Oku. Inter- and Intramolecular Differentiation of Enantiotopic Dioxane Acetals through Oxazaborolidinone-Mediated Enantioselective Ring-Cleavage Reaction: Kinetic Resolution of Racemic 1,3-Alkanediols and Asymmetric Desymmetrization of Meso-1,3-polyols. J. Org. Chem. 2002, 67, 7080. https://doi.org/10.1021/jo025944g
X. Wang, S. Adachi, H. Iwai, H. Takatsuki, K. Fujita, M. Kubo, A. Oku, T. Harada. Enantioselective Lewis Acid-Catalyzed Mukaiyama−Michael Reactions of Acyclic Enones. Catalysis by allo-Threonine-Derived Oxazaborolidinones. J. Org. Chem. 2003, 68, 10046. https://doi.org/10.1021/jo035379x
R. S. Singh, T. Harada. Enantioselective Diels–Alder Reaction of Acyclic Enones Catalyzed byallo-Threonine-Derived Chiral Oxazaborolidinone. Eur. J. Org. Chem. 2005, 3433. https://doi.org/10.1002/ejoc.200500356
S. Simsek, M. Horzella, M. Kalesse. Oxazaborolidinone-Promoted Vinylogous Mukaiyama Aldol Reactions. Org. Lett. 2007, 9, 5637. https://doi.org/10.1021/ol702640w
(a) S. Adachi, T. Harada. Asymmetric Mukaiyama Aldol Reaction of Nonactivated Ketones Catalyzed by allo-Threonine-Derived Oxazaborolidinone. Org. Lett. 2008, 10, 4999. https://doi.org/10.1021/ol802087u (b) S. Adachi, F. Tanaka, K. Watanabe, T. Harada. Oxazaborolidinone-Catalyzed Enantioselective Friedel−Crafts Alkylation of Furans and Indoles with α,β-Unsaturated Ketones. Org. Lett. 2009, 11, 5206. https://doi.org/10.1021/ol9021436
K. Micoine, A. Fürstner. Concise Total Synthesis of the Potent Translation and Cell Migration Inhibitor Lactimidomycin. J. Am. Chem. Soc. 2010, 132, 14064. https://doi.org/10.1021/ja107141p
U. Costantino, F. Fringuelli, M. Nocchetti, O. Piermatti. Chiral borane of layered α-Zirconium-N-(m-solfophenyl)-l-Valine-Phosphonate Methanphosphonate promoters for the asymmetric Mukaiyama Aldol reaction. Appl. Catal., A. 2007, 326, 100. https://doi.org/10.1016/j.apcata.2007.04.007
M. T. Gieseler, M. Kalesse. Asymmetric Vinylogous Mukaiyama Aldol Reaction of Aldehyde-Derived Dienolates. Org. Lett. 2011, 13, 2430. https://doi.org/10.1021/ol2006727
(a) A. Kena Diba, C. Noll, M. Richter, M. T. Gieseler, M. Kalesse. Intramolecular Stereoselective Protonation of Aldehyde-Derived Enolates. Angew. Chem., Int. Ed. 2010, 49, 8367. https://doi.org/10.1002/anie.201004619 (b) W. Kohl, B. Witte, B. Kunze, V. Wray, D. Schomburg, H. Reichenbach, G. Hӧfle. Antibiotika aus Gleitenden Bakterien, XXVII. Angiolam A –ein neues Antibiotikum aus Angiococcus disciformis (Myxobacterales). Liebigs Ann. Chem. 1985, 2088. https://doi.org/10.1002/jlac.198519851016
A. Eggert, C. Etling, L. Millbrodt, G. Schulz, M. Kalesse. Oxazaborolidinone-Mediated Asymmetric Bisvinylogous Mukaiyama Aldol Reaction. Org. Lett. 2021, 23, 22, 8722. https://doi.org/10.1021/acs.orglett.1c03165
Y. Zhang, Y. Liu, Y. Du. First Total Synthesis of 27-Deoxylyngbyabellin A. Synthesis2021, 53, 2874. https://doi.org/10.1055/a-1478-9088